Advertisements
Advertisements
प्रश्न
Write the chemical equation for the preparation of 4 KMnO from 2. MnO Why does purple colour of acidified permanganate solution decolourise when it oxidises Fe2- to Fe3+ ?
उत्तर
Chemical equations for the preparation of KMnO4 from MnO2
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
`3MnO_4^2+4H -> 2MnO_4^- + 2 MnO_4^- + MnO_2 + 2H_2O `
\[\ce{ MnO_2 ->[Fused with oxidised][with air or KNO3]}MnO_4^{2-}\] maganate ion
\[\ce{ \underset{Manganate}{MnO_4^{2-}} ->[Electrolytic oxidation][in alkaline solution] MnO_4^- }\] permanganateion
Purple colour of acidified permanganate solution decolourise when it oxidises Fe2- + Fe3+
In acidic medium, Reduction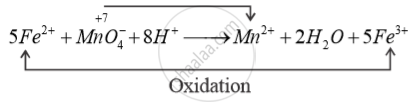
KMnO4 is behaving as oxidising Agent.
So, purple colour of acidified 4 KMnO solutions decolourises.
APPEARS IN
संबंधित प्रश्न
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Complete the following chemical equation
Cr2O72- + 3Sn2+ + 14H+ →
Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
Complete the following equations:
`2MnO_4^(-)+16H^++5S^(2-)rarr`
Name a member of the lanthanoid series that is well-known to exhibit +2 oxidation state.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
