Advertisements
Advertisements
Question
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
Solution
[NiCl4]2-
Ni is in + 2 oxidation state.
3d8 configuration 
Cl- is weak field ligand. So, pairing doesnot occur.
sp3 hybridised orbitals of 2 Ni2+ 

[ ] NiCl is paramagnetic as n = 2
4 [ ( ) ] Ni CO Ni is in ‘0’ oxidation state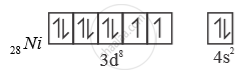
In presence of ‘CO’ pairing of e– takes place ‘CO’ is strong field ligand. So, with ‘CO
[Ni(CO)4] → 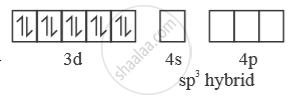
[Ni(CO)4] → 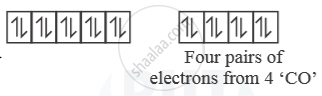
So, [Ni(CO)4] is diamagnetic as n = 0.
APPEARS IN
RELATED QUESTIONS
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Co(NH3)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Is the coordination compound a high spin or low spin complex?
If orbital quantum number (l) has values 0, 1, 2 and 3, deduce the corresponding value of principal quantum number, n.
How many radial nodes for 3p orbital?
Which of the following methods is used for measuring bond length?
Which of the following has square planar structures?
Write the hybridisation and magnetic behaviour of [CoF6]3−.
[Given: Atomic number of Co = 27]
