Advertisements
Advertisements
प्रश्न
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
उत्तर
[NiCl4]2-
Ni is in + 2 oxidation state.
3d8 configuration 
Cl- is weak field ligand. So, pairing doesnot occur.
sp3 hybridised orbitals of 2 Ni2+ 

[ ] NiCl is paramagnetic as n = 2
4 [ ( ) ] Ni CO Ni is in ‘0’ oxidation state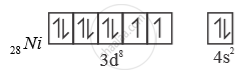
In presence of ‘CO’ pairing of e– takes place ‘CO’ is strong field ligand. So, with ‘CO
[Ni(CO)4] → 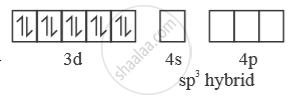
[Ni(CO)4] → 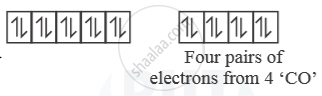
So, [Ni(CO)4] is diamagnetic as n = 0.
APPEARS IN
संबंधित प्रश्न
On the basis of valence bond theory explain the nature of bonding in [CoF6]3 ion.
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Fe(CN)6]4−
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[CoF6]3−
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Cr(H2O)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Write the hybridization and shape of the following complexes:
[Ni(CN)4]2−
If orbital quantum number (l) has values 0, 1, 2 and 3, deduce the corresponding value of principal quantum number, n.
Which of the following has square planar structures?
What is the no. of possible isomers for the octahedral complex [Co(NH3)2(C2O4)2]?
In Fe(CO)5, the Fe – C bond possesses
[Ni(CO)4] has tetrahedral geometry while [Ni(CN)4]2− has square planar, yet both exhibit diamagnetism. Explain.
[Atomic number: Ni = 28]
