Advertisements
Advertisements
Question
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
Solution
| [Ni(CN)4]2− | Ni(28): 4s23d8 |
| Ni2+(28): 4s03d8 |
CN− is a strong field ligand, so it causes the pairing of electrons.
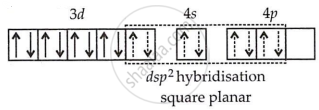
It is diamagnetic due to the absence of unpaired electrons.
In [NiCl4]2−, Cl− is a weak field ligand, so it does not cause electron pairing.
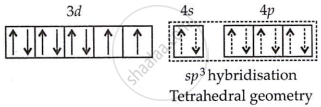
It is paramagnetic due to the presence of unpaired electrons.
APPEARS IN
RELATED QUESTIONS
On the basis of valence bond theory explain the nature of bonding in [CoF6]3 ion.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[FeF6]3−
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[CoF6]3−
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Mn(CN)6]^{3-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Cr(H2O)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Write the hybridization and shape of the following complexes:
[Ni(CN)4]2−
Which of the statement given below is incorrect about H2O2?
As the s-character of hybridised orbital increases, the bond angle
What is the no. of possible isomers for the octahedral complex [Co(NH3)2(C2O4)2]?
In Fe(CO)5, the Fe – C bond possesses
Valence bond theory is based on the assumption that the bonds formed between the metal ions and ligands are ______
Using valence bond theory, predict the hybridization and magnetic character of the following:
[CoF6]3– [Atomic number of Co = 27]
According to the valence bond theory, the hybridization of central metal atom is dsp2 for which one of the following compounds?
Write the hybridisation and magnetic behaviour of [CoF6]3−.
[Given: Atomic number of Co = 27]
During chemistry class, a teacher wrote \[\ce{[Ni(CN)4]^2-}\] as a coordination complex ion on the board. The students were asked to find out the magnetic behaviour and shape of the complex. Pari, a student, wrote the answer paramagnetic and tetrahedral whereas another student Suhail wrote diamagnetic and square planer.
Evaluate Pari’s and Suhail’s responses.
