Advertisements
Advertisements
Question
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Cr(H2O)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Solution
\[\ce{[Cr(H2O)6]^{3+}}\]:
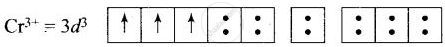
(i) Hybridisation – d2sp3
(ii) Inner orbital complex
(iii) Paramagnetic
(iv) Magnetic moment = `sqrt(3(3 + 2))` = 3.87 B.M
APPEARS IN
RELATED QUESTIONS
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Explain the geometry of `[Co(NH_3)_6]^(3+)` on the basis of hybridisation. (Z of Co = 27)
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Mn(CN)6]^{3-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
The type of hybridization involved in Octahedral complexes is ______.
Which of the statement given below is incorrect about H2O2?
As the s-character of hybridised orbital increases, the bond angle
In Fe(CO)5, the Fe – C bond possesses
According to the valence bond theory, the hybridization of central metal atom is dsp2 for which one of the following compounds?
The magnetic moment of [NiCl4]2− is ______.
[Atomic number: Ni = 28]
