Advertisements
Advertisements
प्रश्न
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
उत्तर
| [Ni(CN)4]2− | Ni(28): 4s23d8 |
| Ni2+(28): 4s03d8 |
CN− is a strong field ligand, so it causes the pairing of electrons.
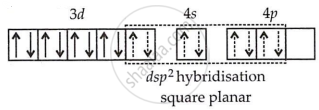
It is diamagnetic due to the absence of unpaired electrons.
In [NiCl4]2−, Cl− is a weak field ligand, so it does not cause electron pairing.
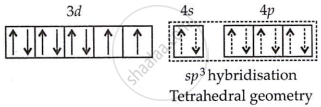
It is paramagnetic due to the presence of unpaired electrons.
APPEARS IN
संबंधित प्रश्न
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Fe(CN)6]4−
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Co(C2O4)3]3−
Explain the geometry of `[Co(NH_3)_6]^(3+)` on the basis of hybridisation. (Z of Co = 27)
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Cr(H2O)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[FeCl6]^{4-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
The type of hybridization involved in Octahedral complexes is ______.
Write the hybridization and shape of the following complexes:
[Ni(CN)4]2−
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Is the coordination compound a high spin or low spin complex?
As the s-character of hybridised orbital increases, the bond angle
Which of the following has square planar structures?
In Fe(CO)5, the Fe – C bond possesses
Valence bond theory is based on the assumption that the bonds formed between the metal ions and ligands are ______
Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6]3-
- type of hybridization
- magnetic moment value
- type of complex – inner, outer orbital complex
According to the valence bond theory, the hybridization of central metal atom is dsp2 for which one of the following compounds?
Write the hybridisation and magnetic behaviour of [CoF6]3−.
[Given: Atomic number of Co = 27]
During chemistry class, a teacher wrote \[\ce{[Ni(CN)4]^2-}\] as a coordination complex ion on the board. The students were asked to find out the magnetic behaviour and shape of the complex. Pari, a student, wrote the answer paramagnetic and tetrahedral whereas another student Suhail wrote diamagnetic and square planer.
Evaluate Pari’s and Suhail’s responses.
