Advertisements
Advertisements
Question
Write the electronic configuration of Fe(III) on the basis of crystal field theory when it forms an octahedral complex in the presence of (i) strong field, and (ii) weak field ligand. (Atomic no.of Fe=26)
Solution
`"Fe"= ["Ar"]3"d"^6 4"s"^2`
`"Fe"^{3+} = ["Ar"]3"d"^6 4"s"^2`
`"Fe"^(+3) = ["AR"] 3"d"^5 4"s"^0`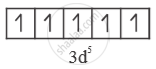
In octahedral crystal field.
(i) Strong field ligand - Electrons will be paired.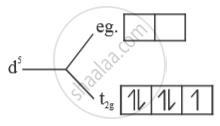
No. of unpaired e- = 1
Paramagnetic
`μ = sqrt(n(n+2) = sqrt(1(3) = sqrt3 B.M`
(ii) Weak field ligand
- Electrons follow Hund’s rule 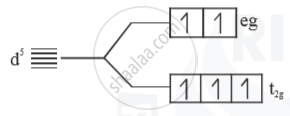
No. of unpaired = 5
Paramagnetic
`μ =sqrt(5(5+2))= sqrt(35) B.M.`
APPEARS IN
RELATED QUESTIONS
Complete and balance the following reactions:
(1) P4 + H2SO4 → ____ + _____ + _____ + _____
(2) Ag + HNO3(dilute) → _____ + ______ + _____ + _____
The CFSE for octahedral \[\ce{[CoCl6]^{4-}}\] is 18,000 cm–1. The CFSE for tetrahedral \[\ce{[CoCl4]^{2-}}\] will be ______.
\[\ce{CuSO4 . 5H2O}\] is blue in colour while \[\ce{CuSO4}\] is colourless. Why?
Match the complex ions given in Column I with the hybridisation and number of unpaired electrons given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Hybridisation, number of unpaired electrons) |
| A. \[\ce{[Cr(H2O)6]^{3+}}\] | 1. dsp2, 1 |
| B. \[\ce{[Co(CN)4]^{2-}}\] | 2. sp3d2, 5 |
| C. \[\ce{[Ni(NH3)6]^{2+}}\] | 3. d2sp3, 3 |
| D. \[\ce{[MnF6]^{4-}}\] | 4. sp3, 4 |
| 5. sp3d2, 2 |
Considering crystal field theory, strong-field ligands such as CN–:
The correct order of increasing crystal field strength in following series:
Crystal field stabilising energy for high spind4 octahedral complex is:-
The correct order of intensity of colors of the compounds is ______.
The complex that has highest crystal field splitting energy (Δ) is ______.
On the basis of Crystal Field theory, write the electronic configuration for the d5 ion with a strong field ligand for which Δ0 > P.
