Advertisements
Advertisements
Question
Why does p-nitrochlorobenzene undergo displacement reactions readily with attack of nucleophilic HOΘ ion?
Solution
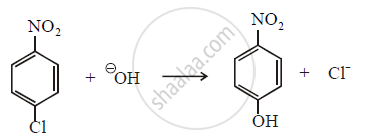
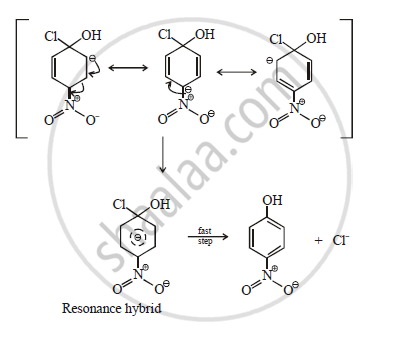
Aryl halides undergo nucleophillic displacement reactions readily when a strong electron withdrawing group like –NO2 is present at ortho or para positions. When p-nitrochlorobenzene reacts with alkali, p-nitrophenol is obtained when –NO2 group at para position with respect to halogen, then mechanism of reaction is as follows.
shaalaa.com
Haloarenes - Nucleophilic Substitution
Is there an error in this question or solution?
