Advertisements
Advertisements
प्रश्न
Why does p-nitrochlorobenzene undergo displacement reactions readily with attack of nucleophilic HOΘ ion?
उत्तर
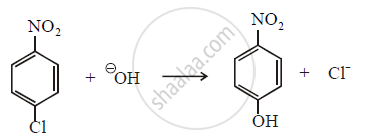
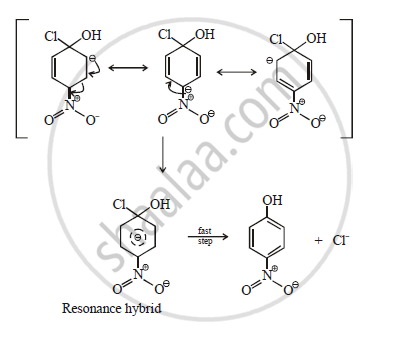
Aryl halides undergo nucleophillic displacement reactions readily when a strong electron withdrawing group like –NO2 is present at ortho or para positions. When p-nitrochlorobenzene reacts with alkali, p-nitrophenol is obtained when –NO2 group at para position with respect to halogen, then mechanism of reaction is as follows.
shaalaa.com
Haloarenes - Nucleophilic Substitution
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
