Advertisements
Advertisements
प्रश्न
What is the action of bromine in alkaline medium on
i. CH3CH2NO2
ii. 
उत्तर
(a)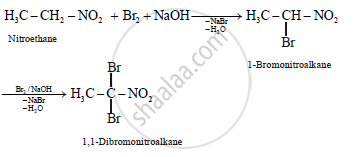
(b)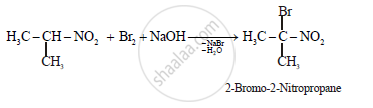
APPEARS IN
संबंधित प्रश्न
How do you convert the following: Chlorobenzene to 2-chlorotoluene
Write a short note on Sandmeyer’s reaction.
The synthesis of alkyl fluorides is best accomplished by ____________.
Conant Finkelstein reaction for the preparation of alkyl iodide is based upon the fact that:
\[\ce{X ->[AgNO3][HNO3] Yellow or While ppt}\]
Which of the following cannot be X?
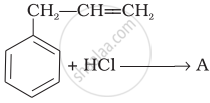
What is ‘A’ in the following reaction?
The boiling points of alcohols are higher than those of hydrocarbons of comparable masses due to ______.
The order of reactivity of alcohols with halogen acids is ______.
(A) \[\ce{CH3CH2 - CH2 - OH}\]
(B) \[\begin{array}{cc}
\phantom{}\ce{CH3CH2 - CH - OH}\\
\phantom{...}\phantom{}|\\
\phantom{......}\ce{CH3}
\end{array}\]
(C) \[\begin{array}{cc}
\phantom{........}\ce{CH3}\\
\phantom{.....}\phantom{}|\\
\phantom{}\ce{CH3CH2 - C - OH}\\
\phantom{.....}\phantom{}|\\
\phantom{........}\ce{CH3}
\end{array}\]
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Why is the solubility of haloalkanes in water very low?
Write down the structure and IUPAC name for neo-pentylbromide.
A hydrocarbon of molecular mass 72 g mol–1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Predict the major product formed when HCl is added to isobutylene. Explain the mechanism involved.
Match the structures given in Column I with the names in Column II.
| Column I | Column II | |
| (i) | 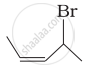 |
(a) 4-Bromopent-2-ene |
| (ii) | 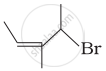 |
(b) 4-Bromo-3-methylpent-2-ene |
| (iii) | 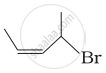 |
(c) 1-Bromo-2-methylbut-2-ene |
| (iv) | 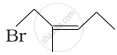 |
(d) 1-Bromo-2-methylpent-2-ene |
Which compound would undergo dehydrohalogenation with strong base to give the alkene shown below as the only alkene product?
CH3 – CH2CH = CH – CH3
Butene-1 may be converted to butane by reaction with
The alky halide is converted into an alcohol by
Which is gem-dihalide?
Benzyl chloride (16H5CH2Cl) can be prepared from toluene by chlorination with
Iodo form can be prepared form all except
The alkyl halide which does not give white precipitate with alcoholic AgNO3 solution is :-
The product of reaction of alcoholic silver nitrite with ethyl bromide are:
Benzoyl chloride is is prepared from benzoic acid by
Predict the reagent for carrying out the following transformations:
Ethanoic acid to 2-chloroethanoic acid
The major product of the following reaction is:
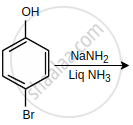
Arrange the following in increasing order of reactivity towards nitration
- p-xylene
- bromobenzene
- mesitylene
- nitrobenzene
- benzene
Choose the correct answer from the options given below:
