Advertisements
Advertisements
प्रश्न
Write a short note on Sandmeyer’s reaction.
उत्तर
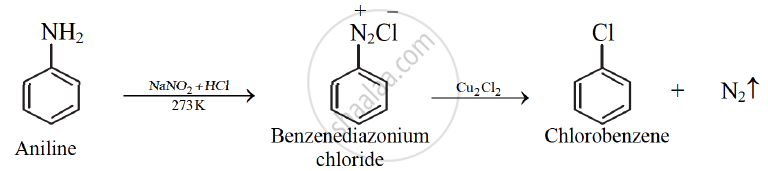
Sandmeyer’s reaction:
When a primary aromatic amine, dissolved or suspended in aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide, results in the replacement of the diazonium group by - Cl or - Br. This reaction is known as Sandmeyer' s reaction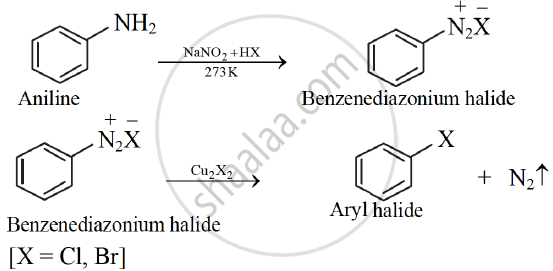
eg.
APPEARS IN
संबंधित प्रश्न
How is propene converted into 1- bromopropane and 2 - bromopropane?
The preparation of alkyl fluoride from alkyl chloride, in presence of metallic fluorides is known as ______________.
How do you convert: Propene to 1-iodopropane
How do you convert the following: Prop-1-ene to 1-fluoropropane
\[\ce{CH3 = CH2CH3 + H - I -> CH3CH2CH2I + CH3CHICH3}\] (major). This reaction is:
Finkelstein reaction is ______.
In the following reaction, the compound used in the reaction for synthesizing ethyl fluoride is:
______ \[\ce{+ AgF -> H3C - F + AgBr}\]
Rectified spirit is a mixture of ____________.
The solution of a chemical compound reacts with AgNO3 solution to form a white precipitate of Y which dissolves in NH4OH to give a complex Z. When Z is treated with dilute HNO3, Y reappears. The chemical compound X can be:
The synthesis of alkyl fluorides is best accomplished by ____________.
Among the following, the most reactive towards alcoholic KOH is:
What is ‘A’ in the following reaction?
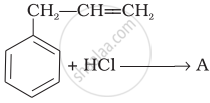
The boiling points of alcohols are higher than those of hydrocarbons of comparable masses due to ______.
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Alkyl halides are prepared from alcohol by treating with ______.
(i) HCl + ZnCl2
(ii) Red P + Br
(iii) H2SO4 + KI
(iv) All the above
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ______ or ______.
(i) CaF2
(ii) CoF2
(ii) Hg2F2
(iv) NaF
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Which of the following is the most stable free radical?
Which compound would undergo dehydrohalogenation with strong base to give the alkene shown below as the only alkene product?
CH3 – CH2CH = CH – CH3
Iodo form can be prepared form all except
The alkyl halide which does not give white precipitate with alcoholic AgNO3 solution is :-
Benzoyl chloride is is prepared from benzoic acid by
\[\begin{array}{cc}
\ce{Ph - CH - CH2 - CH2 ->[Zn - Cu][Δ] Product}\\
\phantom{..}|\phantom{......}|\phantom{....................}\\
\phantom{}\ce{Br}\phantom{....}\ce{Br}\phantom{..................}
\end{array}\]
Product of the above reaction is
Name the possible alkenes which will yield 1-chloro-1-methylcyclohexane on their reaction with HCl. Write the reactions involved.
In the given reactions sequence, the major product 'C' is:
\[\ce{C8H10 ->[HNO3][H2SO4] A ->[Br2][\Delta] B ->[alcoholic][KOH] C}\]
The decreasing order of reactivity of the following organic molecules towards AgNO3 solution is ______.
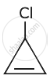

- \[\begin{array}{cc}\ce{CH3CHCH3}\\
|\phantom{..}\\\ce{Cl}\phantom{.}\end{array}\] - \[\begin{array}{cc}\ce{CH3CHCH2NO2}\\
|\phantom{......}\\\ce{Cl}\phantom{.....}\end{array}\]
