Advertisements
Advertisements
प्रश्न
The preparation of alkyl fluoride from alkyl chloride, in presence of metallic fluorides is known as ______________.
विकल्प
Williamson’s reaction
Finkelstein reaction
Swarts reaction
Wurtz reaction
उत्तर
Swarts reaction
Preparation of alkyl fluorides from alkyl chlorides or bromides in presence of metallic fluorides like AgF, Hg2F2 reaction is known as Swarts reaction.
R - X + AgF → R - F + AgX
APPEARS IN
संबंधित प्रश्न
How do you convert the following: Chlorobenzene to 2-chlorotoluene
Draw the structures of major monohalo products in each of the following reactions :
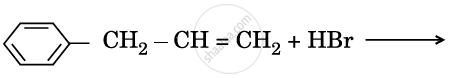
Draw the structure of major monohalo product in each of the following reactions :
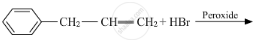
Write a short note on Sandmeyer’s reaction.
\[\ce{CH3 = CH2CH3 + H - I -> CH3CH2CH2I + CH3CHICH3}\] (major). This reaction is:
Rectified spirit is a mixture of ____________.
The solution of a chemical compound reacts with AgNO3 solution to form a white precipitate of Y which dissolves in NH4OH to give a complex Z. When Z is treated with dilute HNO3, Y reappears. The chemical compound X can be:
\[\ce{X ->[AgNO3][HNO3] Yellow or While ppt}\]
Which of the following cannot be X?
The order of reactivity of alcohols with halogen acids is ______.
(A) \[\ce{CH3CH2 - CH2 - OH}\]
(B) \[\begin{array}{cc}
\phantom{}\ce{CH3CH2 - CH - OH}\\
\phantom{...}\phantom{}|\\
\phantom{......}\ce{CH3}
\end{array}\]
(C) \[\begin{array}{cc}
\phantom{........}\ce{CH3}\\
\phantom{.....}\phantom{}|\\
\phantom{}\ce{CH3CH2 - C - OH}\\
\phantom{.....}\phantom{}|\\
\phantom{........}\ce{CH3}
\end{array}\]
Alkyl halides are prepared from alcohol by treating with ______.
(i) HCl + ZnCl2
(ii) Red P + Br
(iii) H2SO4 + KI
(iv) All the above
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ______ or ______.
(i) CaF2
(ii) CoF2
(ii) Hg2F2
(iv) NaF
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Identify the products A and B formed in the following reaction:
\[\ce{CH3 - CH2 - CH = CH - CH3 + HCl -> A + B}\]
Match the structures given in Column I with the names in Column II.
| Column I | Column II | |
| (i) | 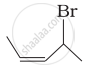 |
(a) 4-Bromopent-2-ene |
| (ii) | 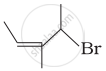 |
(b) 4-Bromo-3-methylpent-2-ene |
| (iii) | 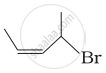 |
(c) 1-Bromo-2-methylbut-2-ene |
| (iv) | 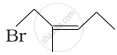 |
(d) 1-Bromo-2-methylpent-2-ene |
Assertion: Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason: Phosphorus chlorides give pure alkyl halides.
Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
Which is gem-dihalide?
Iodo form can be prepared form all except
The alkyl halide which does not give white precipitate with alcoholic AgNO3 solution is :-
The product of reaction of alcoholic silver nitrite with ethyl bromide are:
SN1 and SN2 product are same with
Name the possible alkenes which will yield 1-chloro-1-methylcyclohexane on their reaction with HCl. Write the reactions involved.
Predict the reagent for carrying out the following transformations:
Ethanoic acid to 2-chloroethanoic acid
In Finkelstein Reaction, which reactants are used?
Arrange the following in increasing order of reactivity towards nitration
- p-xylene
- bromobenzene
- mesitylene
- nitrobenzene
- benzene
Choose the correct answer from the options given below:
