Advertisements
Advertisements
Question
The preparation of alkyl fluoride from alkyl chloride, in presence of metallic fluorides is known as ______________.
Options
Williamson’s reaction
Finkelstein reaction
Swarts reaction
Wurtz reaction
Solution
Swarts reaction
Preparation of alkyl fluorides from alkyl chlorides or bromides in presence of metallic fluorides like AgF, Hg2F2 reaction is known as Swarts reaction.
R - X + AgF → R - F + AgX
APPEARS IN
RELATED QUESTIONS
How do you convert: Propene to 1-iodopropane
Write the structure of the major product in each of the following reaction :
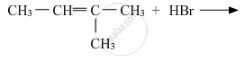
Write the structure of the major product in each of the following reaction :
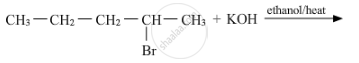
Draw the structures of major monohalo products in each of the following reactions :
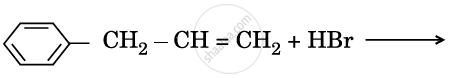
Draw the structure of major monohalo product in each of the following reactions :
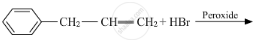
Write a short note on Sandmeyer’s reaction.
The synthesis of alkyl fluorides is best accomplished by ____________.
Gem-dibromide is ____________.
The catalyst used in the preparation of an alkyl chloride by the action of dry HCl on alcohol is ____________.
Conant Finkelstein reaction for the preparation of alkyl iodide is based upon the fact that:
\[\ce{X ->[AgNO3][HNO3] Yellow or While ppt}\]
Which of the following cannot be X?
Which of the following is halogen exchange reaction?
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ______ or ______.
(i) CaF2
(ii) CoF2
(ii) Hg2F2
(iv) NaF
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Which of the following compounds (a) and (b) will not react with a mixture of \[\ce{NaBr}\] and \[\ce{H2SO4}\]. Explain why?
(a) \[\ce{CH3CH2CH2OH}\]
(b) 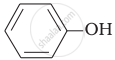
tert-Butylbromide reacts with aq. \[\ce{NaOH}\] by SN1 mechanism while n-butylbromide reacts by SN2 mechanism. Why?
Predict the major product formed when HCl is added to isobutylene. Explain the mechanism involved.
Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
Which of the following is the most stable free radical?
Which compound would undergo dehydrohalogenation with strong base to give the alkene shown below as the only alkene product?
CH3 – CH2CH = CH – CH3
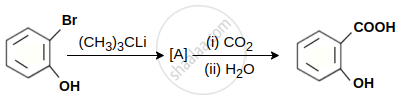
In the given conversion the compound A is:
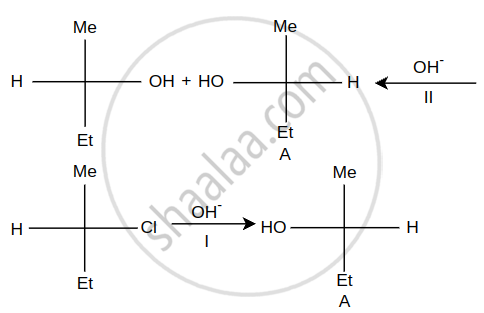
Steps I and II are ______.
The major product of the following reaction is:
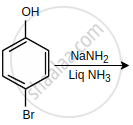
Write the structure of the following organic halogen compound.
1,4-Dibromobut-2-ene
