Advertisements
Advertisements
Question
Which of the following is halogen exchange reaction?
Options
\[\ce{RX + NaI -> RI + NaX}\]
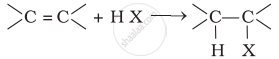
\[\ce{R-OH + HX ->[ZnCl2] R-X + H2O}\]
MCQ
Solution
\[\ce{R X + Nal -> RI + Na X}\]
Explanation:
Alkyl iodides are often prepared by the reaction of alkyl chlorides/bromides with Nal in dry acetone. This reaction is known as the Finkelstein reaction. NaCl or NaBr thus formed is precipitated in dry acetone. It facilitates the forward reaction according to Le chatelier's Principle. According to above/given equation there happens to be 'an exchange of halogens involved between alkyl group (- R) and sodium atom (Na), hence the reaction is very often termed as "halogen exchange reaction".
shaalaa.com
Is there an error in this question or solution?
