Advertisements
Advertisements
Question
Which of the following compounds (a) and (b) will not react with a mixture of \[\ce{NaBr}\] and \[\ce{H2SO4}\]. Explain why?
(a) \[\ce{CH3CH2CH2OH}\]
(b) 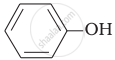
Short Note
Solution

As mixture of \[\ce{NaBr}\] and \[\ce{H2SO4}\] gives \[\ce{Br} \] gas.
\[\ce{2NaBr + 3H2SO4 -> 2NaHSO4 + SO2 + Br2 + 2H2O}\]
Phenol (b) rects with \[\ce{Br2}\] to form 2, 4, 6-tribomophenol.
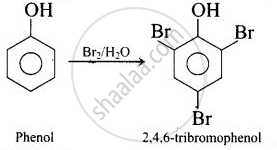
But (a) \[\ce{CH3CH2CH2OH}\] does not react with \[\ce{Br2}\] gas.
shaalaa.com
Is there an error in this question or solution?
Chapter 10: Haloalkanes and Haloarenes - Exercises [Page 143]
