Advertisements
Advertisements
Question
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Short Note
Solution
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenas with chlorine and bromine respectively, in the presence of Lewis acid catalysts like iron or iron chloride.
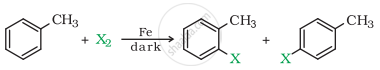
Mechanism: \[\ce{Cl - Cl + FeCl3 -> FeCl4 + Cl+}\]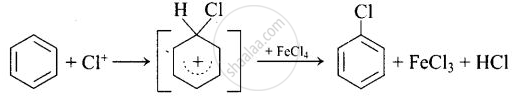
Lewis acid generates the electrophile required for the substitution.
shaalaa.com
Is there an error in this question or solution?
Chapter 10: Haloalkanes and Haloarenes - Exercises [Page 143]
