Advertisements
Advertisements
प्रश्न
How is propene converted into 1- bromopropane and 2 - bromopropane?
उत्तर
The addition of hydrogen halide to an unsymmetrical alkene gives two products.
Propene on reaction with hydrogen bromide forms 80% 2-bromopropane (isopropyl bromide) and 20% 1-bromopropane (n-propyl bromide).

APPEARS IN
संबंधित प्रश्न
How do you convert the following: Prop-1-ene to 1-fluoropropane
Write the structure of the major product in each of the following reaction :
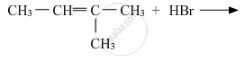
Write a short note on Sandmeyer’s reaction.
Finkelstein reaction is ______.
Rectified spirit is a mixture of ____________.
The solution of a chemical compound reacts with AgNO3 solution to form a white precipitate of Y which dissolves in NH4OH to give a complex Z. When Z is treated with dilute HNO3, Y reappears. The chemical compound X can be:
Gem-dibromide is ____________.
\[\ce{X ->[AgNO3][HNO3] Yellow or While ppt}\]
Which of the following cannot be X?
The order of reactivity of alcohols with halogen acids is ______.
(A) \[\ce{CH3CH2 - CH2 - OH}\]
(B) \[\begin{array}{cc}
\phantom{}\ce{CH3CH2 - CH - OH}\\
\phantom{...}\phantom{}|\\
\phantom{......}\ce{CH3}
\end{array}\]
(C) \[\begin{array}{cc}
\phantom{........}\ce{CH3}\\
\phantom{.....}\phantom{}|\\
\phantom{}\ce{CH3CH2 - C - OH}\\
\phantom{.....}\phantom{}|\\
\phantom{........}\ce{CH3}
\end{array}\]
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Identify the products A and B formed in the following reaction:
\[\ce{CH3 - CH2 - CH = CH - CH3 + HCl -> A + B}\]
A hydrocarbon of molecular mass 72 g mol–1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Which of the following compounds would undergo SN1 reaction faster and why?
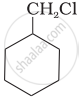 |
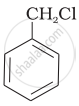 |
| (A) | (B) |
Which of the following is the most stable free radical?
Which compound would undergo dehydrohalogenation with strong base to give the alkene shown below as the only alkene product?
CH3 – CH2CH = CH – CH3
Which of the following species is an odd electron intermediate?
The most stable free radical among the following is
Which is gem-dihalide?
The alkyl halide which does not give white precipitate with alcoholic AgNO3 solution is :-
Benzoyl chloride is is prepared from benzoic acid by
SN1 and SN2 product are same with
\[\begin{array}{cc}
\ce{Ph - CH - CH2 - CH2 ->[Zn - Cu][Δ] Product}\\
\phantom{..}|\phantom{......}|\phantom{....................}\\
\phantom{}\ce{Br}\phantom{....}\ce{Br}\phantom{..................}
\end{array}\]
Product of the above reaction is
The major product of the following reaction is:
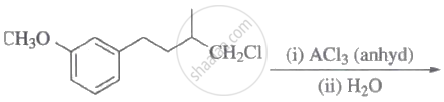
\[\ce{C2H5Cl + AgF -> C2H5F + AgCl}\] The above reaction is called ______.
The major product of the following reaction is:
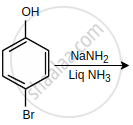
In the given reactions sequence, the major product 'C' is:
\[\ce{C8H10 ->[HNO3][H2SO4] A ->[Br2][\Delta] B ->[alcoholic][KOH] C}\]
Arrange the following in increasing order of reactivity towards nitration
- p-xylene
- bromobenzene
- mesitylene
- nitrobenzene
- benzene
Choose the correct answer from the options given below:
