Advertisements
Advertisements
Question
Write the chemical reaction to prepare novolac polymer.
Solution

APPEARS IN
RELATED QUESTIONS
Write any ‘two' uses of terylene.
Write the reactions involved in the preparation of PVC
Explain the following term: Homopolymers
Write the formulae of the raw materials used for preparation of Dextran.
The Zieglar-Natta catalyst is used in the preparation of _______.
(A) LDPE
(B) PHBV
(C) PAN
(D) HDPE
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
Draw the structures of veronal and thymine.
Choose the correct option from the given alternatives.
Which of the following is made up of polyamides?
Answer the following in one sentence.
Identify 'A' in the following reaction:
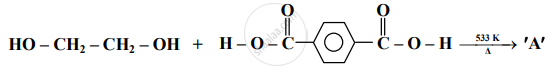
Answer the following in one sentence.
What type of intermolecular force leads to high-density polymer?
Answer the following.
Match the following pairs:
| Name of polymer | Monomer |
| 1. Teflon | a. CH2 = CH2 |
| 2. PVC | b. CF2 = CF2 |
| 3. Polyester | c. CH2 = CHCl |
| 4. Polythene | d. C6H5OH and HCHO |
| 5. Bakelite | e. Dicarboxylic acid and polyhydoxyglycol |
Answer the following.
Draw the structures of polymers formed from the following monomers
H2N–(CH2)5 – COOH
Attempt the following:
What is meant by LDP and HDP? Mention the basic difference between the same with suitable examples.
Attempt the following:
Write preparation, properties and uses of Teflon.
Write the name of the catalyst used for preparation of high density polythene polymer.
write the structure of the monomer used in natural rubber.
Write preparation of low density polythene.
Mention two uses of LDP.
Write chemical reaction for preparation of the following.
Buna-S
Write two uses and two properties of polythene.
Explain vulcanization of rubber.
Define rubber.
The following structure represents the polymer:
\[\begin{array}{cc}
\ce{[-C-CH2-NH-C-(-CH2)5 NH -]_{{n}}}\\
\phantom{}||\phantom{.............}||\phantom{................}\\
\phantom{}\ce{O}\phantom{.............}\ce{O}\phantom{................}
\end{array}\]
Which among the following polymers is used for making handles of cooker?
Which of the following polymer is used in paints?
How many isoprene units are present in abscisic acid?
Which of the following polymers is a heteropolymer?
Which among the following polymers can NOT be remoulded?
Which of the following catalysts is used in preparation of terylene?
Which of the following compounds is used to prepare orlon?
Which among the following polymers is obtained from CH2 = CH – CN by polymerisation?
Which among the following catalysts is used in the preparation of dacron?
Which of the following polymers is prepared by using phenol?
Which of the following polymer is used to make blankets?
Identify addition polymer from the following.
Which of the following polymers is used as insulation for cables?
The commercial name of polyacrylonitrile is ______.
Phenol and formaldehyde undergo condensation to give a polymar (A) which on heating with formaldehyde gives a thermosetting polymer (B). Name the polymers. Write the reactions involved in the formation of (A). What is the structural difference between two polymers?
Which of the following products is formed when benzaldehyde is treated with CH3MgBr and the addition product so obtained is subjected to acid hydrolysis?
F2C = CF2 is monomer of the polymer -
Which of the following polymer has ester linkage?
Trans - form of poly isoprene is:-
Name and draw the structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
How the Bakelite is prepared? Give the steps involved in the preparation.
Name the compound which reacts with formaldehyde to produce ethyl alcohol.
Write the structure of isoprene and the polymer obtained from it.
Write the structure and name of monomer of Nylon-6.
Write the structure and name of monomer of Natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
