Advertisements
Advertisements
Question
Explain vulcanization of rubber.
Solution
Vulcanization of rubber:
- The process by which a network of cross-links is introduced into an elastomer is called vulcanization.
- Vulcanization of rubber is carried out to improve the physical properties of natural rubber.
- The profound effect of vulcanization enhances the properties like tensile strength, stiffness, elasticity, toughness, etc. of natural rubber.
- Most frequently the used process of vulcanization is sulfur vulcanization. Sulfur forms crosslinks between polyisoprene chains which results in improved properties of natural rubber.
APPEARS IN
RELATED QUESTIONS
Based on molecular forces, what type of polymer is neoprene?
The Zieglar-Natta catalyst is used in the preparation of _______.
(A) LDPE
(B) PHBV
(C) PAN
(D) HDPE
Write the structure of melamine.
Write the monomers of the following polymer :
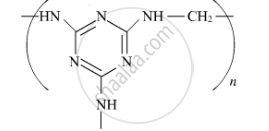
Choose the correct option from the given alternatives.
Which of the following is made up of polyamides?
Answer the following in one sentence.
Identify 'A' in the following reaction:
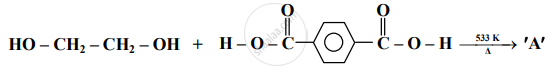
Answer the following in one sentence.
Identify 'B' in the following reaction:
\[\ce{H2N -(CH2)6 - NH2 + HOOC - (CH2)4 - COOH ->[N2][533 K]}\]'B'
Answer the following in one sentence.
What type of intermolecular force leads to high-density polymer?
Answer the following in one sentence.
Identify thermoplastic and thermosetting plastic from the following:
- PET
- Urea formaldehyde resin
- Polythene
- Phenol formaldehyde resin
Answer the following.
Write the reaction of the formation of Nylon 6.
Draw the structures of polymers formed from the following monomers
\[\ce{n HOOC–R–COOH + n HO–R'–OH}\]
Answer the following.
Draw the structures of polymers formed from the following monomers
H2N–(CH2)5 – COOH
Name and draw structure of the repeating unit in natural rubber.
Attempt the following:
Explain the vulcanisation of rubber. Which vulcanizing agents are used for the following synthetic rubber?
a. Neoprene
b. Buna-N
Write the structure of isoprene and the polymer obtained from it.
Nylon 6, 6 is a condensation polymer of hexamethylenediamine and _____________
Monomer used for preparation of polyacrylonitrile is _____________
Write a chemical reaction for the preparation of the following polymer.
polyacrylonitrile
Write preparation of low density polythene.
Write the name of one example of each polymer in which following repeating units.
\[\begin{array}{cc}
\ce{(-CF2-CF2-), -[NH-(CH2)5-CO] -, -(CH2-CH-), (-CH2-CH2-)}\\
\phantom{............................}|\\
\phantom{..............................}\ce{CN}
\end{array}\]
Write the name and formulae of the monomers used for the preparation of dacron.
Write chemical reactions for the preparation of high-density polythene.
Explain the reactions involved in the preparation of viscose rayon.
Which among the following polymers is obtained from styrene and 1-3-butadiene?
Select the CORRECT match for both the polymers.
Which among the following polymers is obtained from CH2 = CH – CN by polymerisation?
Which among the following polymers is an example of addition polymer?
Which of the following polymers is used as insulation for cables?
Which among the following is an example of addition polymer?
Which of the following is not a semisynthetic polymer?
Name the polymers used in laminated sheets and give the name of monomeric units involved in its formation.
Which of the following polymers is synthesized using a free radical polymerisation technique?
Which of the following polymers do not involve cross linkages?
Which of the following polymer is used for manufacturing of buckets, dustbins, pipes, etc?
Which among the following polymers has high tensile strength and is used to obtain tyre cords?
Identify the monomer used to prepare neoprene.
Which of the following is a polymer of enzyme?
The monomer of natural rubber is ______.
Answer the following.
Name and draw structure of the repeating unit in natural rubber.
Answer the following.
Name and draw structure of the repeating unit in natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
How the Bakelite is prepared? Give the steps involved in the preparation.
Name the compound which reacts with formaldehyde to produce ethyl alcohol.
Write the structure of isoprene and the polymer obtained from it.
Name and draw the structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
