Advertisements
Advertisements
Question
Write the name of one example of each polymer in which following repeating units.
\[\begin{array}{cc}
\ce{(-CF2-CF2-), -[NH-(CH2)5-CO] -, -(CH2-CH-), (-CH2-CH2-)}\\
\phantom{............................}|\\
\phantom{..............................}\ce{CN}
\end{array}\]
Solution
- \[\ce{(-CF2-CF2-)}\] is monomer unit of Teflon polymer.
- \[\ce{-[NH-(CH2)5-CO] -}\] is monomer unit of nylon 6 polymer.
- \[\begin{array}{cc}\ce{-(CH2-CH-)}\\\phantom{....}|\\\phantom{......}\ce{CN}
\end{array}\] is monomer unit of polyacrylonitrile polymer. - \[\ce{(-CH2-CH2-)}\] is monomer unit of polyethylene polymer.
APPEARS IN
RELATED QUESTIONS
Write the reactions involved in the preparation of PVC
Bakelite is the polymer of:
(a) Benzaldchyde and phenol
(b) Acetaldehyde and phenol
(c) Formaldehyde and phenol
(d) Formaldehyde and benzyl alcohol
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
Draw the structures of veronal and thymine.
Answer the following in one sentence.
Identify thermoplastic and thermosetting plastic from the following:
- PET
- Urea formaldehyde resin
- Polythene
- Phenol formaldehyde resin
Answer the following.
Write the reaction of the formation of Nylon 6.
Answer the following.
Draw the structures of polymers formed from the following monomers
H2N–(CH2)5 – COOH
Name and draw structure of the repeating unit in natural rubber.
Answer the following.
Write name and formula of raw material from which bakelite is made.
Identify condensation polymers and addition polymers from the following.
-(CH2 - CH = CH - CH2 -)n
Attempt the following:
Write the chemical reactions involved in the manufacture of Nylon 6,6.
Attempt the following:
What is meant by LDP and HDP? Mention the basic difference between the same with suitable examples.
Attempt the following:
Write preparation, properties and uses of Teflon.
Write the structure of isoprene and the polymer obtained from it.
Write the name of the catalyst used for preparation of high density polythene polymer.
Monomers ethylene glycol and terephthalic acid undergo condensation polymerization to give polymer called ___________
Write chemical reaction for preparation of the following.
Buna-S
Write chemical reaction for preparation of the following.
Neoprene
Define rubber.
The following structure represents the polymer:
\[\begin{array}{cc}
\ce{[-C-CH2-NH-C-(-CH2)5 NH -]_{{n}}}\\
\phantom{}||\phantom{.............}||\phantom{................}\\
\phantom{}\ce{O}\phantom{.............}\ce{O}\phantom{................}
\end{array}\]
Identify additional polymers from the following.
I. \[\begin{array}{cc}
\ce{-(CH2 - CH -)_{{n}}}\\
\phantom{....}|\\
\phantom{.......}\ce{C6H5}
\end{array}\]
II. \[\ce{-(CH2 - CH = CH - CH2 -)_{{n}}}\]
III. \[\ce{-(CO(CH2)4 - CONH(CH2)6NH -)_{{n}}}\]
IV.
![]()
How many isoprene units are present in abscisic acid?
The INCORRECT match for the polymer with its application is:
Which of the following is the monomer of neoprene?
Which of the following polymers is a heteropolymer?
Which of the following catalysts is used in preparation of terylene?
Which of the following compounds is used to prepare orlon?
Which among the following polymers is obtained from CH2 = CH – CN by polymerisation?
Which of the following is not a semisynthetic polymer?
Match the polymers given in Column I with their repeating units given in Column II.
| Column I | Column II |
| (i) Acrilan |
(a) \[\begin{array}{cc} |
| (ii) Polystyrene | (b) \[\begin{array}{cc} \ce{Cl}\phantom{.......}\\ |\phantom{........}\\ \phantom{}\ce{-(CH2 - C = CH - CH2)\underset{n}{-}} \end{array}\] |
| (iii) Neoprene | (c) \[\begin{array}{cc} \phantom{................................}\ce{CN}\\ \phantom{..............................}|\\ \ce{-(CH2 - CH = CH - CH2 - CH2 - CH)\underset{n}{-}} \end{array}\] |
| (iv) Novolac | (d) \[\begin{array}{cc} \ce{-(CH2 - CH)\underset{n}{-}}\\ \phantom{.....}|\\ \phantom{.......}\ce{CN} \end{array}\] |
| (v) Buna—N | (e) 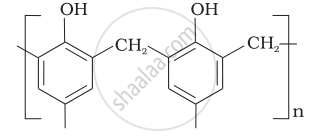 |
| (f) \[\begin{array}{cc} \ce{-(CH2 - CH)\underset{n}{-}}\\ \phantom{.....}|\\ \phantom{......}\ce{Cl} \end{array}\] |
Phenol and formaldehyde undergo condensation to give a polymar (A) which on heating with formaldehyde gives a thermosetting polymer (B). Name the polymers. Write the reactions involved in the formation of (A). What is the structural difference between two polymers?
Which of the following products is formed when benzaldehyde is treated with CH3MgBr and the addition product so obtained is subjected to acid hydrolysis?
Which of the following polymer has ester linkage?
Which one of the following polymers are prepared by addition polymerization?
The catalyst used for the polymerisation of olefins is ______.
Which among the following polymers has high tensile strength and is used to obtain tyre cords?
Identify the monomer used to prepare neoprene.
Which of the following is a polymer of enzyme?
Answer the following.
Write the structure of isoprene and the polymer obtained from it.
Answer the following.
Name and draw structure of the repeating unit in natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
How the Bakelite is prepared? Give the steps involved in the preparation.
Name the compound which reacts with formaldehyde to produce ethyl alcohol.
Write the structure and name of monomer of Nylon-6.
Write the structure of isoprene and the polymer obtained from it.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Write the structure of isoprene and the polymer obtained from it.
