Advertisements
Advertisements
Questions
Write a short note on Hoffmann bromamide degradation.
Explain Hoffmann bromamide degradation reaction
Solution 1
(a)Hoffmann Bromanide degradation
(1) The conversion of amides into amine in presence of bromine and alkali is known on Hoffmann degradation of amides.
(2) An important characteristic of this reaction is that on amine with one carbon less than those in the amide is formed.
(3) This reaction is an example of molecular rearrangement and involves migration of an alkyl or aryl group from the carbonyl carbon to this adjacent nitrogen atom.
Example:
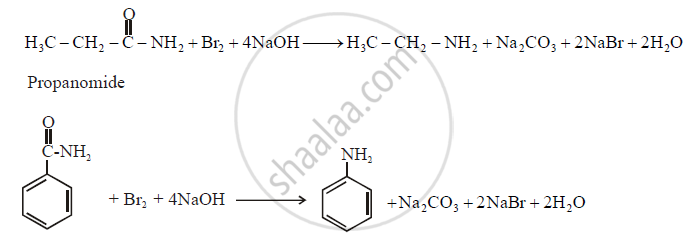
Solution 2
Hoffmann bromamide reaction:
a) Primary amine can be prepared by reaction of amide with bromine and aqueous or
alcoholic sodium hydroxide.
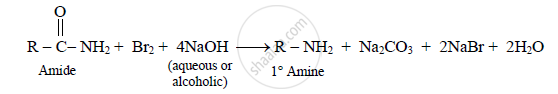
eg. Ethanamine is prepared by reaction of propanamide with bromine and aqueous or
alcoholic sodium hydroxide.
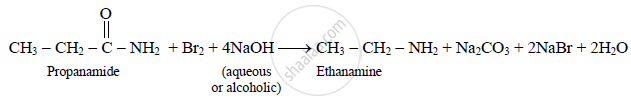
b) This reaction is known as Hoffmann bromamide degradation. It involves molecular
rearrangement. An alkyl or aryl group migrates from the carbonyl carbon to the
adjacent nitrogen atom.
c) This reaction is useful for decreasing the length of carbon chain by one carbon atom.
RELATED QUESTIONS
Write short notes on the following Hofmann’s bromamide reaction
Give the structures of A, B and C in the following reaction:

Give the structures of A, B and C in the following reaction:
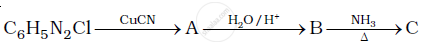
Give the structures of A, B and C in the following reaction:
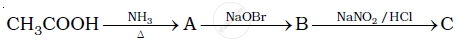
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Write the reactions of aromatic with nitrous acid.
Write the reactions of aliphatic primary amines with nitrous acid.
Mention 'two' uses of propan-2-one.
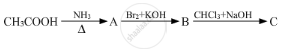
Give the structures of A, B and C in the following reactions :
Give the structures of A, B and C in the following reactions :
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Write structures of compounds A and B in each of the following reactions: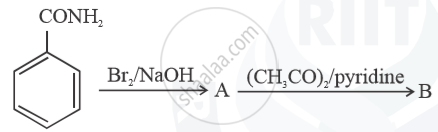
Answer the following
Identify A and B in the following reactions.
\[\ce{C6H5CH2Br->[alco.][KCN]A ->[Na/ethanol]B.}\]
Answer the following
Write a reaction to convert acetic acid into methylamine.
Write reactions to prepare ethanamine from Acetonitrile.
The following amines is the product of Gabriel phthalimide synthesis.
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
Acetamide on reduction using Na/C2H5OH gives ____________.
The end product C of the following reaction is
\[\ce{C2H5NH2 ->[HNO2] A ->[PCl5] B ->[NH3][Alcohol] C}\]
What product is formed when \[\ce{R - C ≡ N}\] is hydrolysed?
\[\ce{CH3-CN ->[Na/C2H5OH]}\]
The product formed is ____________.
Which of the following amines forms a clear solution when treated with benzene sulphonyl chloride and excess of potassium hydroxide?
Which of the following reagents is used in Mendius reduction reaction of alkyl cyanide?
Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Alkyl halides are insoluble in water.
Reason (R): Alkyl halides have halogen attached to sp3 hybrid carbon.
Select the most appropriate answer from the options given below:
Amongst the given set of reactants, the most appropriate for preparing 2° amine is ______.
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
Which of the following compounds is the weakest Brönsted base?
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) \[\ce{Sn/HCl}\]
(ii) \[\ce{Fe/HCl}\]
(iii) \[\ce{H2 - Pd}\]
(iv) \[\ce{Sn/NH4OH}\]
Which of the following reactions are correct?
(i)
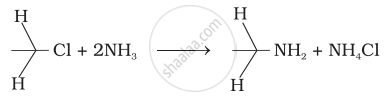
(ii)
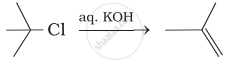
(iii)
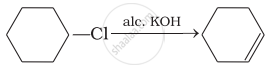
(iv)
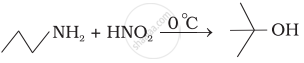
Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(i) Acetyl chloride/pyridine followed by reaction with conc.\[\ce{H2SO4 }\] + conc. \[\ce{HNO3}\].
(ii) Acetic anyhdride/pyridine followed by conc.\[\ce{H2SO4}\] + conc.\[\ce{HNO3}\].
(iii) Dil. HCl followed by reaction with conc.\[\ce{H2SO4}\] + conc.\[\ce{HNO3}\].
(iv) Reaction with conc.\[\ce{HNO3}\] + conc.\[\ce{H2 SO4}\].
What is the best reagent to convert nitrile to primary amine?
Write following conversions:
nitrobenzene `->` acetanilide
How will you carry out the following conversion?
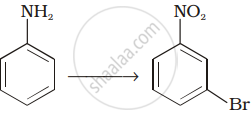
How will you carry out the following conversions?
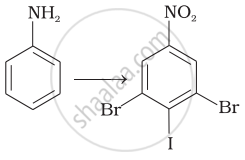
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
Describe Gabriel's phthalimide synthesis. (Give reaction)
Ethylamine can be prepared by the action of bromine and caustic potash on which compound?
C6H5CONHCH3 can be converted into C6H5CH2NHCH3 by:-
A compound 'A' on reduction with iron scrap and hydrochloric acid gives compound 'B' with molecular formula C6H7N. Compound 'B' on reaction with CHCl3 and alcoholic KOH produces an obnoxious smell of carbylamine due to the formation of 'C'. Identify 'A', 'B' and 'C' and write the chemical reactions involved.
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
Which of the following amines can be prepared by Gabriel phthalimide reaction?
Identify the product ‘C’ in the following reaction.
\[\ce{Aniline ->[(CH3CH)2O][Pyridine] A ->[Br2][CH3COOH] B ->[H^+ or OH^-] C}\]
Which of the following would not be a good choice for reducing nitrobenzene to aniline?
Amides can be converted into amines by the reaction named ______.
Identify A and B in the following reaction.
\[\ce{C6H5CH2Br ->[Alco.][KCN] A ->[Na/Ethanol][reduction] B}\]
Write a short note on the following:
Ammonolysis
Write the name of reduction product formed when ethyl cyanide is treated with sodium and alcohol.
