HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2017-2018
Date & Time: 28th February 2018, 11:00 am
Duration: 3h
Advertisements
The process in which the value of ΔU = 0 is __
Adiabatic
Isothermal
Isobaric
Isochoric
Chapter: [0.03] Chemical Thermodynamics and Energetic
An ionic crystal lattice has `r^+/r` radius ratio of 0.320, its coordination number is __
(a) 3
(b) 4
(c) 6
(d) 8
Chapter: [0.01] Solid State
In the hydrogen-oxygen fuel cell, the carbon rods are immersed in the hot aqueous solution of __
(a) KCl
(b) KOH
(c) H2SO4
(d) NH4Cl
Chapter: [0.04] Electrochemistry
The chemical formula of willemite is __
(a) ZnS
(b) ZnCO3
(c) ZnO
(d) Zn2SiO4
Chapter: [0.06] General Principles and Processes of Isolation of Elements
The oxidation state of nitrogen in dinitrogen trioxide is ____
(a) + 1
(b) + 2
(c) + 3
(d) + 4
Chapter: [7.01] Group 15 Elements
Which of the following 0.1 M will aqueous solutions exert highest osmotic pressure?
(a) `Al_2(SO_4)_3`
(b) `Na_2SO_4`
(c) `MgCl_2`
(d) KCl
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
The half-life period of zero order reaction A → product is given by
(a) `([A]_0)/k`
(b) `0.693/k`
(c) `[A]_0/(2k)`
(d) `(2[A]_0)/k`
Chapter: [0.05] Chemical Kinetics [0.06] Chemical Kinetics
Derive the relation between the elevation of boiling point and molar mass of solute.
Chapter: [0.02] Solutions and Colligative Properties
State Third law of thermodynamics. Give ‘two’ uses.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Give two uses of the Third law of thermodynamics.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Draw a neat and labelled diagram of the lead storage battery.
Chapter: [0.04] Electrochemistry
Explain Ionic solids are hard and brittle.
Chapter: [0.01] Solid State [0.01] Solid State
A certain reaction occurs in the following steps-
\[\ce{Cl_g + O3(g)->ClO(g) +O2(g)}\]
\[\ce{ClO_g + O(g)->Cl(g) +O2(g)}\]
a) What is the molecularity of each of the elementary steps?
b) Identify the reaction intermediate and write the chemical equation for the overall reaction.
Chapter:
Define Semipermeable membrane
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Define Reference electrode
Chapter: [0.05] Electrochemistry
What is the action of chlorine on CS2
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
Answer the following.
What is the action of chlorine on Excess NH3.
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
Advertisements
Write the chemical equations involved in van Arkel method for refining zirconium metal.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Write balanced chemical equations for Phosphorus reacts with magnesium
Chapter: [7.01] Group 15 Elements
Write balanced chemical equations for Flowers of sulphur boiled with calcium hydroxide
Chapter: [7.01] Group 15 Elements
Write balanced chemical equations for Action of ozone on hydrogen peroxide.
Chapter: [7.01] Group 15 Elements
The density of iron crystal is 8.54-gram cm–3. If the edge length of the unit cell is 2.8 A° and atomic mass is 56 gram mol–1, find the number of atoms in the unit cell. (Given: Avogadro's number = 6.022x1023,1A° = 1x10-8 cm)
Chapter: [0.01] Solid State
How many faradays of electricity are required to produce 13 gram of aluminium from aluminium chloride solution? (Given: Molar mass of Al = 27.0-gram mol–1)
Chapter: [0.04] Electrochemistry
Calculate the internal energy at 298K for the formation of one mole of ammonia, if the enthalpy change at constant pressure is – 42.0 kJ mol-1.
(Given: R = 8.314 J K-1 mol-1)
Chapter: [0.03] Chemical Thermodynamics and Energetic
Define the Enthalpy of atomization
Chapter: [0.03] Chemical Thermodynamics and Energetic
Define Enthalpy of vaporization
Chapter: [0.03] Chemical Thermodynamics and Energetic
Draw the structure of IF7. Write its geometry and the type of hybridization.
Chapter: [7.03] Group 17 Elements
State Henry’s law.
Chapter: [0.02] Solutions and Colligative Properties
22.22 gram of urea was dissolved in 300 grams of water. Calculate the number of moles of urea and molality of the urea solution. (Given: a Molar mass of urea = 60 gram mol-1)
Chapter: [0.02] Solutions and Colligative Properties
What is the action of carbon on the following metal oxides:
Fe2O3 in the blast furnace
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the action of carbon on the following metal oxides :
ZnO in the vertical retort furnace
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Write the molecular and structural formulae of thiosulfuric acid.
Chapter: [7.02] Group 16 Elements
Write the molecular and structural formulae of Dithionous acid
Chapter: [7.02] Group 16 Elements
The reaction A + B → products is first order in each of the reactants
1) How does the rate of reaction change if the concentration of A is increased by factor 3?
2) What is the change in the rate of reaction if the concentration of A is halved and concentration of B is doubled?
Chapter:
A polymer used in paints is
(a) Nomex
(b) Thiokol
(c) Saran
(d) Glyptal
Chapter:
The number of primary and secondary hydroxyl groups in ribose are -
(a) 1, 3
(b) 2, 3
(c) 3, 1
(d) 3, 2
Chapter: [14.01] Carbohydrates
The ligand diethylene triamine is -
(a) monodentate
(b) bidentate
(c) tridentate
(d) tetradentate
Chapter: [0.09] Coordination Compounds
Propene on oxidation with diborane in presence of alkaline hydrogen peroxide gives
(a) propane-1- ol
(b) propane-2-ol
(c) allyl alcohol
(d) propane-1, 2-diol
Chapter: [11.01] Alcohols
Baeyer’s reagent is -'
(a) acidified potassium dichromate
(b) alkaline potassium dichromate
(c) alkaline potassium permanganate
(d) acidified potassium permanganate
Chapter: [8.01] D-block Elements
Identify 'A' in the following reaction -
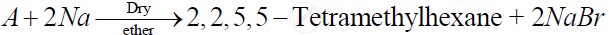
(a) 2- Bromo-2 methylbutane
(b) 1 -Bromo-2,2-dimethylpropane
(c) 1 - Bromo - 3 -methylbutane
(d) 1 - Bromo- 2 -methylpropane
Chapter: [10.01] Haloalkanes
Advertisements
An antifertility drug is -
(a) Novestrol
(b) Histamine
(c) Vernal
(d) Equanil
Chapter: [16.01] Chemicals in Medicines
Write balanced chemical equations for the conversion of `CrO_4^(2-)` to `Cr_2O_7^(2-)` in acidic medium and `Cr_2O_7^(2-)` to `CrO_4^(2-)`
in basic medium.
Chapter: [8.01] D-block Elements
Explain the geometry of `[Co(NH_3)_6]^(3+)` on the basis of hybridisation. (Z of Co = 27)
Chapter: [0.09] Coordination Compounds
Why ethanol has the higher boiling point than ethane?
Chapter: [11.01] Alcohols
Write only reactions for the preparation of benzophenone from benzonitrile.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
What is the action of p-toluenesulphonychloride on ethylamine and diethylamine?
Chapter: [13.01] Amines
Write the correct reaction for formation of the peptide bond between amino acids
Chapter: [14.02] Proteins
Explain the term Antiseptics
Chapter: [16.01] Chemicals in Medicines
Explain only reaction mechanism for alkaline hydrolysis of tert-butyl bromide
Chapter: [10.02] Haloarenes
Complete and rewrite the balanced chemical equations

Chapter: [0.1] Halogen Derivatives [10.01] Haloalkanes
Complete and rewrite the balanced chemical equations
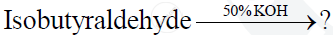
Chapter: [12.02] Carboxylic Acids
Complete and rewrite the balanced chemical equations
Butanone + 2, 4-dinitro-phenylhydrazine ![]()
Chapter: [12.02] Carboxylic Acids
How carbolic acid is prepared from benzene sulphonic acid ?
Chapter: [11.02] Phenols
Write a chemical equation for the action of neutral ferric chloride on phenol.
Chapter: [11.01] Alcohols
Explain the preparation and uses of nylon-2-nylon-6.
Chapter: [0.15] Polymers
How is glucose prepared from cane sugar?
Chapter: [14.01] Carbohydrates
Write the formula of the complex copper (II) hexacyanoferrate (II).
Chapter: [14.01] Carbohydrates
What is Lanthanoid contraction?
Chapter: [8.02] F-block Elements
Explain the cause of Lanthanoids contraction.
Chapter: [8.02] F-block Elements
Draw the structures of chloroxylenol
Chapter: [16.01] Chemicals in Medicines
Draw the structures of adenine.
Chapter: [14.04] Nucleic Acids
How are ethylamine and ethyl methyl amine distinguished by using nitrous acid?
Chapter: [13.01] Amines
What is the action of the following reagents on ethanoic acid?
1) `LiAlH_4"/"H_3O^+`
2) `PCl_3 , "heat"`
3) `P_2O_5, "heat"`
Chapter: [12.01] Aldehydes and Ketones
Identify 'A' and 'B' in the following reaction and rewrite the complete reaction :
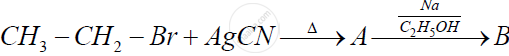
Chapter: [0.13] Amines [13.01] Amines
Write a short note on Hoffmann bromamide degradation.
Chapter: [0.13] Amines [13.01] Amines
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2017 - 2018
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2018 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
