Advertisements
Advertisements
Question
What is the action of carbon on the following metal oxides :
ZnO in the vertical retort furnace
Solution
An action of Carbon on ZnO
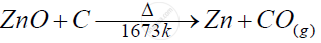
APPEARS IN
RELATED QUESTIONS
What is calcination?
Explain calcination with reactions.
Define Hydrometallurgy?
What is the process in which concentrated ore is reduced to the corresponding metal by heating at high temperature with a reducing agent?
(a) Polling
(b) Pyrometallurgy
(c) Hydrometallurgy
(d) Calcination
Which solution is used for the leaching of silver metal in the presence of air in the metallurgy of silver?
Reduction of metal oxide to metal becomes easier if the metal obtained is in liquid state. Why?
Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
MnO2 and Ca3(PO4)2 present in iron ore get reduced to Mn and P in the zone of _______.
(A) combustion
(B) reduction
(C) fusion
(D) slag formation
Which one of the following oxidation state of Manganese is unstable?
(a) + 2
(b) + 4
(c) + 5
(d) + 7
What happens when HCl is added to MnO2? Write the equations involved
What is the action of carbon on the following metal oxides:
Fe2O3 in the blast furnace
An element X (molar mass = 60 g mol−1) has a density of 6.23 g cm−3. Identify the type of cubic unit cell, if the edge length of the unit cell is 4 ✕ 10−8 cm.
Account for the following :
Reducing character decreases from SO2 to TeO2.
Which metal is also found in sea beds?
In the extraction of chlorine by electrolysis of brine ______.
Which of the following reactions is an example of autoreduction?
Brine is electrolysed by using inert electrodes. The reaction at anode is ______.
At temperatures above 1073 K coke can be used to reduce \[\ce{FeO}\] to \[\ce{Fe}\]. How can you justify this reduction with Ellingham diagram?
Why are sulphide ores converted to oxide before reduction?
Which of the following reactions is an example of auto reduction?
