Advertisements
Advertisements
Question
Complete and rewrite the balanced chemical equations

Solution
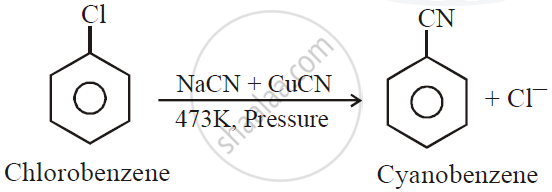
APPEARS IN
RELATED QUESTIONS
Write the structure of the following compound:
1-Chloro-4-ethylcyclohexane
Write the structure of the following compound:
1,4-Dibromobut-2-ene
Give the IUPAC name of the following compound:
CH3CH(Cl)CH(Br)CH3
Give the IUPAC name of the following compound:
CHF2CBrClF
Give the IUPAC name of the following compound:
ClCH2C≡CCH2Br
Give the IUPAC name of the following compound:
CH3C(p-ClC6H4)2CH(Br)CH3
Write the structure of the following organic halogen compound.
p-Bromochlorobenzene
Write the structure of the following organic halogen compound.
2-Bromobutane
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
How are the following conversions carried out?
2-methylbutan-1-ol into 2 -methylbutanoic acid.
IUPAC name of is C6H5 − CH2 − C − CH2 − CH2 − CH2 − CH3 is _______
(A) 1-Phenylhexan-2-one
(B) 6-Phenylhexan-5-one
(C) 1-Benzylhexan-5-one
(D) Dodecan-5-one
How is nitromethane prepared from the following?
alkyl halide
Using IUPAC norms write the formulae for the following:
Potassium trioxalatochromate (III)
Give IUPAC names of the following compound:
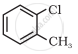
IUPAC name of (CH3)3CCl:
IUPAC name of CH3CH2C(Br) = CH–Cl is ____________.
Benzene hexachloride is ____________.
Which of the following is most stable?
\[\ce{CH3CH2CH2Cl ->[alc. KOH] B ->[HBr] C ->[Na/ether] D}\]
In the above reaction, the product D is:
In the following sequence of reactions:
\[\ce{C2H5Br ->[AgCN] X ->[Reduction] Y}\]; Y is
Identify Z in the series:
\[\ce{CH2 = CH2 ->[HBr] X ->[aq. KOH] Y ->[Na2CO3][I2 excess] Z}\]
\[\begin{array}{cc}
\ce{CH3-CH-CH=CH2 + HBr -> A}\\
|\phantom{.....................}\\
\ce{CH3}\phantom{..................}
\end{array}\]
'A' is:
Which of the following compounds are gem-dihalides?
(i) Ethylidene chloride
(ii) Ethylene dichloride
(iii) Methylene chloride
(iv) Benzyl chloride
Arrange the following compounds in order of decreasing acidity:
| (I) | 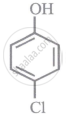 |
| (II) | 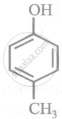 |
| (III) | 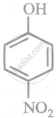 |
| (IV) | 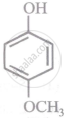 |
Write the structure of the following compound:
1,4-Dibromobut-2-ene
Write the structure of the following organic halogen compound.
4-tert. Butyl-3-iodoheptane
Give the IUPAC name of the following compound:
\[\ce{(CCl3)3CCl}\]
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structures of the following organic halogen compounds.
1,4-Dibromobut-2-ene
Give the IUPAC names of the following compound:
(CCl3)3CCl
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Give the IUPAC name of the following compound:
\[\ce{(CCl3)3CCl}\]
Write the structure of the following organic halogen compound.
1,4-Dibromobut-2-ene
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following compound:
4-tert-Butyl-3-iodoheptane
Give the IUPAC name of the following compound:
\[\ce{(CCl3)3CCl}\]
Write the structure of the organic halogen compound.
1,4-Dibromobut-2-ene
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following compound:
1,4-Dibromobut-2-ene
Write the structure of the following organic halogen compound.
1,4-Dibromobut-2-ene
Write structure of the following compound:
4-tert. Butyl-3-iodoheptane
Give the IUPAC name of the following compound:
\[\ce{(CCl3)3CCl}\]
Write the structure of the following organic halogen compound.
4 – tert – Butyl -3 -iodoheptane
Write structure of the following compound:
1,4-Dibromobut-2-ene
Write structure of the following compound:
4-tert. Butyl-3-iodoheptane
Give the IUPAC name of the following compound:
\[\ce{(CCl3)3CCl}\]
Write the structure of the following organic halogen compound:
1,4-Dibromobut 2 ene
Name the following halide according to the IUPAC system and classify it as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(Cl)(C2H5)CH2CH3}\]
Write the structure of the following compound:
1,4-Dibromobut-2-ene
