Advertisements
Advertisements
Question
How many faradays of electricity are required to produce 13 gram of aluminium from aluminium chloride solution? (Given: Molar mass of Al = 27.0-gram mol–1)
Solution
AlCl3 ![]() Al+3 + 3Cl-
Al+3 + 3Cl-
Al+3 + 3e- ![]() Al
Al
1 mole of Al requires passage of 3 moles of electrons. The charge on 3 moles of e- is 3 Faraday.
Moles of Al produced = `"mass of Al"/"molar mass of Al"`
= `13/27`
= 0.48 moles
As 3F of electricity produces 1 mole of Al
∴ No. of faradays of electricity required to produce 0.48 mole of Al
= 0.48 x 3
= 1.44 Faraday
APPEARS IN
RELATED QUESTIONS
How much electricity in terms of Faraday is required to produce 20 g of \[\ce{Ca}\] from molten \[\ce{CaCl2}\]?
(Given: Molar mass of Calcium is 40 g mol−1.)
State the first law of electrolysis
Following reactions occur at cathode during the electrolysis of aqueous sodium chloride solution:
Na+(aq) + e− ⟶ Na (s) E0 = 2.71 V
H+(aq) + e− ⟶ `1/2` H2 (g) E0 = 0.00 V
On the basis of their standard reduction electrode potential (E0) values, which reaction is feasible at the cathode and why?
If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Suggest a list of metals that are extracted electrolytically.
Consider the reaction: \[\ce{Cr2O^{2-}_7 + 14H^+ + 6e^- -> 2Cr^{3+} + 7H2O}\]
What is the quantity of electricity in coulombs needed to reduce 1 mol of \[\ce{Cr2O^{2-}_7}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{Al^{3+}}\] to \[\ce{Al}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{Cu^{2+}}\] to \[\ce{Cu}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
Write any two uses of H2SO4
Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given : Molar mass of Ag = 108 g mol−1 lF = 96500 C mol−1)
How much quantity of electricity in coulomb is required to deposit 1.346 × 10-3 kg of Ag in 3.5 minutes from AgNO3 solution?
( Given: Molar mass of Ag is 108 × 10-3 kg mol-1 )
Solve the following question.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol–1, Zn = 65.3 g mol–1, 1F = 96500 C mol–1)
Electrolytic cell uses electrical energy to bring about ____________.
What will happen during the electrolysis of aqueous solution of \[\ce{CuSO4}\] by using platinum electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will deposit at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will dissolve at anode.
What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will dissolve at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at anode.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Consider the figure and answer the following question.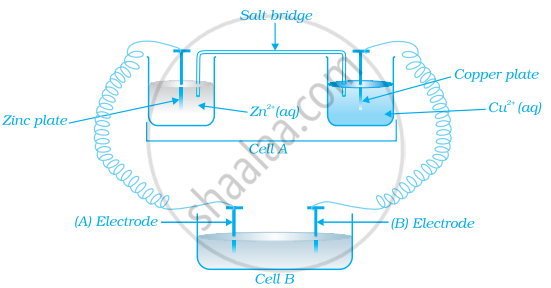
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Time Required to deposite one millimole of aluminium metal by the passage of 9.65 ampere through aqueous solution of aluminium is
What is the quantity of electricity in Coulombs required to produce 4.8 g of Mg from molten MgCl2? How much Ca will be produced if the same amount of electricity was passed through molten CaCl2? (Atomic mass of Mg = 24 u, atomic mass of Ca = 40 u).
A current of 4 amp was passed for 2 hours through a solution of copper sulphate when 5.0 g of copper was deposited. The current efficiency is ______% (Cu = 63.5).
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes hydrogen gas is released at the cathode.
Reason (R): The electrode potential of Cu2+/Cu is greater than that of H+/H2.
Select the most appropriate answer from the options given below:
How much electricity in terms of Faraday is required to produce 40.0 g of \[\ce{Al}\] from molten \[\ce{Al2O3}\]?
(Given: Molar mass of Aluminium is 27 g mol−1.)
