Advertisements
Advertisements
Question
Consider the reaction: \[\ce{Cr2O^{2-}_7 + 14H^+ + 6e^- -> 2Cr^{3+} + 7H2O}\]
What is the quantity of electricity in coulombs needed to reduce 1 mol of \[\ce{Cr2O^{2-}_7}\]?
Solution
According to the given reaction,
\[\ce{Cr2O^{2-}_7}\] One mole of ions requires 6 moles of electrons.
∴ F = 6 × 96500 C
= 579000 C
∴ 579000 C of electricity will be required for the reduction of Cr3+.
APPEARS IN
RELATED QUESTIONS
The charge of how many coulomb is required to deposit 1.0 g of sodium metal (molar mass 23.0 g mol-1) from sodium ions is -
- 2098
- 96500
- 193000
- 4196
How much electricity in terms of Faraday is required to produce 20 g of \[\ce{Ca}\] from molten \[\ce{CaCl2}\]?
(Given: Molar mass of Calcium is 40 g mol−1.)
State the first law of electrolysis
Number of faradays of electricity required to liberate 12 g of hydrogen is:
Suggest a list of metals that are extracted electrolytically.
How much charge is required for the following reduction:
1 mol of \[\ce{Cu^{2+}}\] to \[\ce{Cu}\]?
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
A solution of \[\ce{Ni(NO3)2}\] is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of \[\ce{Ni}\] is deposited at the cathode?
On passing 1.5 F charge, the number of moles of aluminium deposited at cathode are _______ [Molar mass of Al = 27 gram mol–1]
(A) 1.0
(B) 13.5
(C) 0.50
(D) 0.75
Draw neat labelled diagram of electrolytic refining of blister copper
State second law of electrolysis
Explain Faraday’s second law of electrolysis
Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given : Molar mass of Ag = 108 g mol−1 lF = 96500 C mol−1)
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
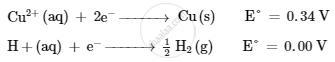
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
How much quantity of electricity in coulomb is required to deposit 1.346 × 10-3 kg of Ag in 3.5 minutes from AgNO3 solution?
( Given: Molar mass of Ag is 108 × 10-3 kg mol-1 )
Solve the following question.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol–1, Zn = 65.3 g mol–1, 1F = 96500 C mol–1)
Electrolytic cell uses electrical energy to bring about ____________.
In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?
A current of 4 amp was passed for 2 hours through a solution of copper sulphate when 5.0 g of copper was deposited. The current efficiency is ______% (Cu = 63.5).
