Advertisements
Advertisements
Question
Draw neat labelled diagram of electrolytic refining of blister copper
Solution

APPEARS IN
RELATED QUESTIONS
On calculating the strength of current in amperes if a charge of 840C (coulomb) passes through an electrolyte in 7 minutes, it will be
- 1
- 2
- 3
- 4
The charge of how many coulomb is required to deposit 1.0 g of sodium metal (molar mass 23.0 g mol-1) from sodium ions is -
- 2098
- 96500
- 193000
- 4196
Number of faradays of electricity required to liberate 12 g of hydrogen is:
Following reactions occur at cathode during the electrolysis of aqueous sodium chloride solution:
Na+(aq) + e− ⟶ Na (s) E0 = 2.71 V
H+(aq) + e− ⟶ `1/2` H2 (g) E0 = 0.00 V
On the basis of their standard reduction electrode potential (E0) values, which reaction is feasible at the cathode and why?
Suggest a list of metals that are extracted electrolytically.
How much charge is required for the following reduction:
1 mol of \[\ce{MnO^-_4}\] to \[\ce{Mn^{2+}}\]?
A solution of \[\ce{Ni(NO3)2}\] is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of \[\ce{Ni}\] is deposited at the cathode?
On passing 1.5 F charge, the number of moles of aluminium deposited at cathode are _______ [Molar mass of Al = 27 gram mol–1]
(A) 1.0
(B) 13.5
(C) 0.50
(D) 0.75
Write any two uses of H2SO4
What is the ratio of volumes of H2 and O2 liberated during electrolysis of acidified water?
(A) 1 : 2
(B) 2 : 1
(C) 1 : 8
(D) 8 : 1
State second law of electrolysis
How many faradays of electricity are required to produce 13 gram of aluminium from aluminium chloride solution? (Given: Molar mass of Al = 27.0-gram mol–1)
Following reactions occur at cathode during the electrolysis of aqueous copper(II) chloride solution :
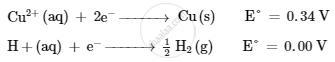
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why ?
Solve the following question.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol–1, Zn = 65.3 g mol–1, 1F = 96500 C mol–1)
What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(i) Copper will deposit at cathode.
(ii) Copper will dissolve at anode.
(iii) Oxygen will be released at anode.
(iv) Copper will deposit at anode.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
Consider the figure and answer the following question.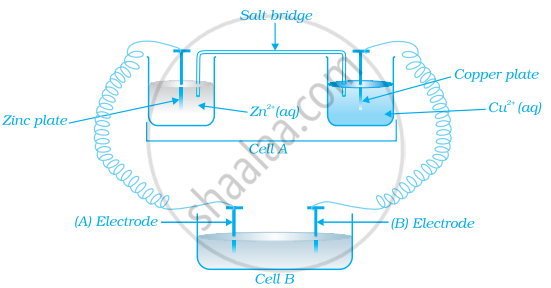
Cell ‘A’ has ECell = 2V and Cell ‘B’ has ECell = 1.1V which of the two cells ‘A’ or ‘B’ will act as an electrolytic cell. Which electrode reactions will occur in this cell?
Time Required to deposite one millimole of aluminium metal by the passage of 9.65 ampere through aqueous solution of aluminium is
The quantity of electricity needed to separately electrolyse 1 M solution of ZnSO4, AlCl3, and AgNO3 completely is in the ratio of ______.
Through an aqueous solution of an unknown salt of metal M (M = 200 g/mol) a current of 1.93 A is passed for 50 min. If 4 g of metal is produced at cathode. The charge on metal ion in solution is ______.
A current of 4 amp was passed for 2 hours through a solution of copper sulphate when 5.0 g of copper was deposited. The current efficiency is ______% (Cu = 63.5).
On passing electricity through nitrobenzene solution, it is converted into azobenzene. The mass of azobenzene is ______ mg, if the same quantity of electricity produces oxygen just sufficient to burn 96 mg of fullerene (C60)·
Assertion (A): During electrolysis of aqueous copper sulphate solution using copper electrodes hydrogen gas is released at the cathode.
Reason (R): The electrode potential of Cu2+/Cu is greater than that of H+/H2.
Select the most appropriate answer from the options given below:
How much electricity in terms of Faraday is required to produce 40.0 g of \[\ce{Al}\] from molten \[\ce{Al2O3}\]?
(Given: Molar mass of Aluminium is 27 g mol−1.)
