HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2015-2016
Date: July 2016
Advertisements
Schottky defects are observed in which solid among the following?
(a) Brass
(b) Cesium Chloride
(c) Zinc sulphide
(d) Stainless steel
Chapter: [0.01] Solid State
No machine has an efficiency unity', is stated in-
(a) first law of thermodynamics
(b) second law of thermodynamics
(c) third law of thermodynamics
(d) Hess' law of constant heat summation
Chapter: [0.03] Chemical Thermodynamics and Energetic
Which among the following reactions is an example of a zero order reaction?
a)
b)
c)
d)
Chapter: [0.05] Chemical Kinetics [0.06] Chemical Kinetics
Potential of saturated calomel electrode is-
0.242 V
1.1 V
0.337 V
0.28 v
Chapter: [0.04] Electrochemistry
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Chapter: [0.09] Coordination Compounds
Which among the following minerals does NOT contain aluminium?
(a) Cryolite
(b) Siderite
(c) China clay
(d) Corundum
Chapter: [0.06] General Principles and Processes of Isolation of Elements
The group 15 element having inner electronic configuration as of argon is-
(a) Phosphorous (z = 15)
(b) Antimony (z =51)
(c) Arsenic (z = 33)
(d) Nitrogen (z = 7)
Chapter: [7.01] Group 15 Elements
Write chemical reactions involved in Van Arkel method for refining Titanium
Chapter: [0.03] Chemical Thermodynamics and Energetic
Explain the relationship between Gibb's standard energy change of the reaction and equilibrium constant.
Chapter: [0.03] Chemical Thermodynamics and Energetic
A face centred cube (FCC) consists of how many atoms? Explain
Chapter: [0.01] Solid State
Describe isolation method in determination of rate law and order of reaction.
Chapter:
Explain the following methods to protect metals from corrosion:
Galvanization
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Explain the following methods to protect metals from corrosion:
Passivation
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Write the Nemst equation and explain the terms involved.
Chapter: [0.04] Electrochemistry
Advertisements
What happens when dilute sulphuric acid is treated with Fe
Chapter: [7.02] Group 16 Elements
What happens when dilute sulphuric acid is treated with Fe
Chapter: [7.02] Group 16 Elements
What happens when dilute sulphuric acid is treated with CaF2
Chapter: [7.02] Group 16 Elements
What happens when dilute sulphuric acid is treated with CaF2
Chapter: [7.02] Group 16 Elements
Explain the term osmosis.
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Define Freezing point.
Chapter: [0.02] Solutions and Colligative Properties
The rate constant of a first order reaction are 0.58 S-1 at 313 K and 0.045 S-1 at 293 K. What is the energy of activation for the reaction?
Chapter: [0.05] Chemical Kinetics
Calculate the standard enthalpy of the reaction, 2C(graphite) + 3H2(g) → C2H6(g), ΔH° = ?
From the following ΔH° values
a)
b)
c) C(graphite) + O2(g) -> CO2(g). ΔH° = -393.5kJ
Chapter: [0.03] Chemical Thermodynamics and Energetic
3.795 g of sulphur is dissolved in 100g of CS2. This solution boils at 319.81 K. What is the molecular formula of sulphur in solution? The boiling point of CS2 is 319.45 K. (Given that Kb for CS2 = 2.42 K kg mol-1 and atomic mass of S = 32)
Chapter: [7.02] Group 16 Elements
How is nitric acid prepared by Ostwald’s process?
Chapter: [7.01] Group 15 Elements
State Third law of thermodynamics. Give ‘two’ uses.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Write applications of standard molar entropy°.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Draw neat labelled diagram of electrolytic refining of blister copper
Chapter: [0.04] Electrochemistry
Determine the density of Cesium chloride which crys tallizes in BCC type structure with the edge length 412.1 pm. The atomic masses of Cs and Cl are 133 and 35.5 respectively.
Chapter:
Predict the co-ordination No. of cs+ ion if
Chapter: [0.09] Coordination Compounds
What happens when thin copper leaves are thrown in jar containing chlorine?
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
H2O is liquid while H2S is gas at room temperature. Explain.
Chapter: [7.02] Group 16 Elements
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
Chapter: [0.04] Electrochemistry
State Henry’s law.
Chapter: [0.02] Solutions and Colligative Properties
Sodium acetate reacts with Ethanoyl chloride to form
(a) Acetic acid
(b) Acetone
(c) Acetic anhydride
(d) Sodium formate
Chapter:
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
What is effective atomic number of Fe (z = 26) in [Fe(CN)6]4-?
(a) 12
(b) 30
(c) 26
(d) 36
Chapter: [8.01] D-block Elements
Maltose is a
(a) Polysaccharide
(b) Disaccharide
(c) Trisaccharide
(d) Monosaccharide
Chapter: [14.01] Carbohydrates
Advertisements
Which one of the following oxidation state of Manganese is unstable?
(a) + 2
(b) + 4
(c) + 5
(d) + 7
Chapter: [0.06] General Principles and Processes of Isolation of Elements
IUPAC name of the following compound is

(a) 3 - Bromo- 3, 4- dimethylheptane
(b) 3, 4- dimethyl - 3- bromoheptane
(c) 5- Bromo- 4, 5- dimethylheptane
(d) 4, 5- dimethyl- 5- bromoheptane
Chapter: [0.09] Coordination Compounds
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Write IUPAC names of the following compounds
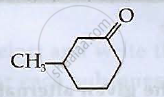
Chapter: [0.09] Coordination Compounds
Write IUPAC names of the following compounds:
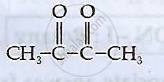
Chapter: [0.09] Coordination Compounds
What are the sources of Vitamin C and Vitamin K?
Chapter: [14.03] Vitamins
Write four points of distinction between Lanthanoids and Actinoids.
Chapter: [0.08] Transition and Inner Transition Elements [8.02] F-block Elements
How is Benzonitrile converted to Benzophenone?
Chapter: [0.09] Coordination Compounds
Write the formulae of the raw materials used for preparation of Buna-S
Chapter: [0.15] Polymers
Write the formulae of the raw materials used for preparation of Dextran.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Write a note on Sandmeyer's reaction.
Chapter: [13.03] Diazonium Salts
What is the action of benzene diazonium chloride on phenol in alkaline medium
Chapter: [13.03] Diazonium Salts
What is the action of benzene diazonium chloride on Aniline
Chapter: [13.03] Diazonium Salts
Explain any two chemical methods of food preservation.
Chapter: [16.02] Chemicals in Food
What is the action of following reagents on glucose?
bromine water
Chapter: [14.01] Carbohydrates
What is the action of following reagents on glucose?
dilute HNO3
Chapter: [14.01] Carbohydrates
What is the action of following reagents on glucose?
hydroxyl amine
Chapter: [14.01] Carbohydrates
What are ligands?
Chapter: [0.09] Coordination Compounds
Write four postulates of Werner's theory.
Chapter: [0.09] Coordination Compounds
Write reactions involved in preparation of potassium dichromate from chrome iron ore
Chapter: [0.08] Transition and Inner Transition Elements
What is metamerism?
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
What is the action of hot HI on it?
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
How are the following polymers prepared?
Orlon
Chapter: [0.15] Polymers
Write the reactions involved in the preparation of Teflon
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Classify the following drugs into Analgesics and Antibiotics
(1) Ofloxacin
(2) Morphine
(3) Ampicillin
(4) Chloramphenicol
Chapter: [16.01] Chemicals in Medicines
Identify 'A' and 'B' and rewrite the reactions
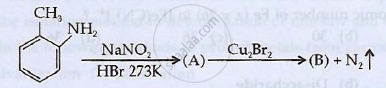
Chapter: [0.09] Coordination Compounds
Identify 'A' and 'B' and rewrite the reactions

Chapter: [0.09] Coordination Compounds
Identify 'A' and 'B' and rewrite the reactions
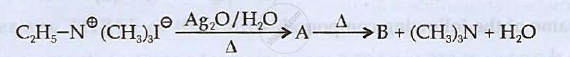
Chapter: [0.09] Coordination Compounds
How are the following conversions carried out?
2-methylbutan-1-ol into 2 -methylbutanoic acid.
Chapter: [0.1] Halogen Derivatives [10.01] Haloalkanes
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
Chapter: [12.02] Carboxylic Acids
How are the following conversions carried out?
Benzoic acid into metanitrobenzoic acid.
Chapter: [0.09] Coordination Compounds
What is the action of Benzene Sulphonyl Chloride on primary, secondary and tertiary
amines?
Chapter: [13.01] Amines
Write two uses of formaldehyde
Chapter: [12.01] Aldehydes and Ketones
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2015 - 2016
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2016 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
