Advertisements
Advertisements
Question
What is the action of following reagents on glucose?
dilute HNO3
Solution
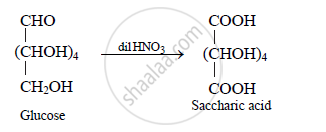
Action of dil. HNO3 on glucose: The oxidation of glucose with dilute nitric acid gives
saccharic acid (a dicarboxylic acid). Hence, a primary alcoholic group (CH2OH) is
present in glucose.
APPEARS IN
RELATED QUESTIONS
What is the action of following reagents on glucose?
bromine water
What is the action of following reagents on glucose?
hydroxyl amine
Stachyose is an example of _______.
(A) monosaccharides
(B) disaccharides
(C) trisaccharides
(D) tetrasaccharides
How is glucose prepared by commercial method? How is peptide linkage formed?
How is glucose prepared by commercial method? How is peptide linkage formed?
How is glucose prepared from starch?
Hydrolysis of starch yields ____________.
Which among the following is the simplest sugar?
Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives ______.
Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a:
(i) monosaccharide
(ii) disaccharide
(iii) reducing sugar
(iv) non-reducing sugar
In nucleoside a base is attached at 1′ position of sugar moiety. Nucleotide is formed by linking of phosphoric acid unit to the sugar unit of nucleoside. At which position of sugar unit is the phosphoric acid linked in a nucleoside to give a nucleotide?
Under what conditions glucose is converted to gluconic and saccharic acid?
How do you explain the presence of five – OH groups in glucose molecule?
How do you explain the presence of an aldehydic group in a glucose molecule?
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its
In the acetylation of glucose, which group is involved in the reaction
Which of the following is an example of aldo hexose?
A nucleoside is made of:-
Which of the following base is not present in RNA?
When sucrose is heated with cone. HNO3 the product is ______.
Which of the following statements is not true about glucose?
How is glucose prepared on commercial scale?
