Advertisements
Advertisements
Question
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
Solution
The radius ratio (r+/r–) defines the coordination number of the cation
`r_(Cs^+) = 1.69 Å, r_(Cl^-) = 1.81 Å`
`∴ r_(Cs^+)/r_(Cl^-) = 1.69/1.89 = 0.9337`
Since, radius ratio is greater than 0.732, the coordination number of cation (Cs+) is 8.
APPEARS IN
RELATED QUESTIONS
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
(NH4)2[CoF4]
IUPAC name of the following compound is

(a) 3 - Bromo- 3, 4- dimethylheptane
(b) 3, 4- dimethyl - 3- bromoheptane
(c) 5- Bromo- 4, 5- dimethylheptane
(d) 4, 5- dimethyl- 5- bromoheptane
Write IUPAC names of the following compounds
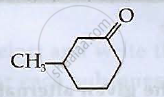
Write applications of co-ordination compounds in medicine and electroplating.
Following compounds are given to you :
2-Bromopentane, 2-Bromo-2-methylbutane, 1-Bromopentane
1) Write the compound which is most reactive towards SN2 reaction.
2) Write the compound which is optically active.
3) Write the compound which is most reactive towards β-elimination reaction.
Complete the following reactions
NH3+3Cl2(excess) ---->
Write the IUPAC names of the following coordination compounds:
`[PtCl_2(NH_3)_4][PtCl_4]`
The ligand triethylenetetramine is _______.
What is the coordination number of chromium in \[\ce{[Cr(NH3)2(H2O2)2]Cl3}\]?
Which of the following complexes formed by \[\ce{Cu^2+}\] ions is most stable?
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is ______.
The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
Which one of the following does not achieve an octet of electrons in the central atom?
Oxidation number of cobalt in K[Co(CO)4] is
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
According to IUPAC nomenclatures, sodium nitroprusside is named as
Metal attached with EDTA in an octahedral complex, has coordination number ______.
Ethylene diamine tetraacetate (EDTA) ion is ______.
How many ions are produced from the complex Co(NH3)6]Cl2 in solution?
The following ligand is:
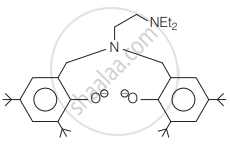
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
In the complex ion \[\ce{[CoCl(en)2ONO]+}\], the coordination number and the oxidation number of the central metal ion are ______ and ______.
Explain the following, giving two examples:
Ligand
Explain the following, giving two examples:
Coordination polyhedron
Explain the following, giving two examples:
Homoleptic
Explain the following, giving two examples:
Heteroleptic
