Advertisements
Advertisements
Question
Write applications of co-ordination compounds in medicine and electroplating.
Solution
Applications of coordination compounds:
1) In medicine:
- Cisplatin [PtCl2(NH3)2] is useful in treatment of cancer.
- EDTA is useful in treatment of poisoning by lead.
2) In electroplating
Stable complexes with very small dissociation in solution are used for electroplating with silver and gold.
- The complex K[Ag(CN)2] is used for electroplating with silver
- The complex K[Au(CN)2] is used for electroplating with gold
APPEARS IN
RELATED QUESTIONS
Explain the following, giving two examples:
Coordination entity
What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
Amongst the following, the most stable complex is:
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Write IUPAC names of the following compounds
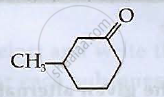
Write IUPAC names of the following compounds:
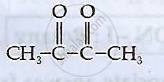
How is Benzonitrile converted to Benzophenone?
Write IUPAC name of the following Complex [Cr(NH3)3Cl3]
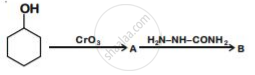
Write structures of compounds A and B of the following reaction :
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
The oxidation number of Fe in K4[Fe(CN)6] is ____________.
Which of the following is non-ionizable?
Which of the following complexes are heteroleptic?
(i) \[\ce{[Cr(NH3)6]^{3+}}\]
(ii) \[\ce{[Fe(NH3)4]Cl2]^+}\]
(iii) \[\ce{[Mn(CN)6]^{4-}}\]
(iv) \[\ce{[Co(NH3)4]Cl2]}\]
Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
Oxidation number of cobalt in K[Co(CO)4] is
The nature of hybridisation in the ammonia molecule is
Which one of the following ligands forms a chelate?
The complex which has no d electrons in the central atom is:-
How many ions are produced from the complex Co(NH3)6]Cl2 in solution?
Given below are two statements.
- Statement I: In CuSO4 · 5H2O, Cu-O bonds are present.
- Statement II: In CuSO4 · 5H2O, ligands coordinating with Cu(II) ion are O- and S based ligands.
In light of the above statement, choose the correct answer from the options given below.
The equivalents of ethylene diamine required to replace the neutral ligands from the coordination sphere of the trans-complex of CoCl3.4NH3 is ______. (Round off to the Nearest Integer).
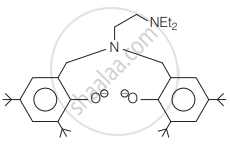
The following ligand is:
Does ionization isomer for the following compound exist? Justify your answer.
\[\ce{Hg[Co(SCN)4]}\]
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Coordination polyhedron
What is meant by didentate ligand?
Give two examples of ambidentate ligand.
