Advertisements
Advertisements
प्रश्न
Write applications of co-ordination compounds in medicine and electroplating.
उत्तर
Applications of coordination compounds:
1) In medicine:
- Cisplatin [PtCl2(NH3)2] is useful in treatment of cancer.
- EDTA is useful in treatment of poisoning by lead.
2) In electroplating
Stable complexes with very small dissociation in solution are used for electroplating with silver and gold.
- The complex K[Ag(CN)2] is used for electroplating with silver
- The complex K[Au(CN)2] is used for electroplating with gold
APPEARS IN
संबंधित प्रश्न
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
What is meant by unidentate ligand?
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
[Mn(H2O)6]SO4
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
IUPAC name of the following compound is

(a) 3 - Bromo- 3, 4- dimethylheptane
(b) 3, 4- dimethyl - 3- bromoheptane
(c) 5- Bromo- 4, 5- dimethylheptane
(d) 4, 5- dimethyl- 5- bromoheptane
How is Benzonitrile converted to Benzophenone?
What happens when PCl5 is heated? Write the equations involved.
Write the structures of compounds A, B and C in the following reactions
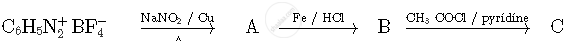
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
The coordination number of the central ion may be obtained from:
Which of the following is non-ionizable?
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is ______.
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
A complex of the type \[\ce{[M(AA)2X2]^{n+}}\] is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Assertion: \[\ce{Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2}\] are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
Oxidation number of cobalt in K[Co(CO)4] is
Which one of the following ligands forms a chelate?
What are Homoleptic complexes?
The equivalents of ethylene diamine required to replace the neutral ligands from the coordination sphere of the trans-complex of CoCl3.4NH3 is ______. (Round off to the Nearest Integer).
What is a chelate complex? Give one example.
Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Coordination number
Give two examples of unidentate ligand.
What is meant by didentate ligand?
