Advertisements
Advertisements
प्रश्न
What is meant by didentate ligand?
उत्तर
A didentate ligand, also known as a bidentate ligand, is a ligand that can form coordination bonds to a metal ion using two donor atoms. These donor atoms are typically separated by a specific number of atoms in the ligand's structure, forming a chelate complex.
संबंधित प्रश्न
Explain the following, giving two examples:
Coordination entity
IUPAC name of the following compound is

(a) 3 - Bromo- 3, 4- dimethylheptane
(b) 3, 4- dimethyl - 3- bromoheptane
(c) 5- Bromo- 4, 5- dimethylheptane
(d) 4, 5- dimethyl- 5- bromoheptane
Write IUPAC names of the following compounds:
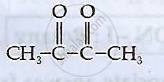
Write the IUPAC names of the following coordination compounds:
`[PtCl_2(NH_3)_4][PtCl_4]`
Write structures of compounds A and B of the following reaction :

The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
Which of the following is an ionic ligand?
Which one of the following ligands forms a chelate?
The oxidation number d-arbitral occupation and co-ordination number of Cr in the complex cis [Cr(en)2Cl2]Cl are respectively.
The complex which has no d electrons in the central atom is:-
The most stable ion is:-
Which of the following ligands can exhibit linkage isomerism?
Glycinato ligand is ______.
Which of the following species cannot act as a ligand? Give reason.
What is meant by the chelate effect? Give an example.
In the complex ion \[\ce{[CoCl(en)2ONO]+}\], the coordination number and the oxidation number of the central metal ion are ______ and ______.
Explain the following, giving two examples:
Coordination number
Give two examples of ambidentate ligand.
