Advertisements
Advertisements
प्रश्न
Give two examples of didentate ligand.
उत्तर
- Ethane-1, 2-diamine (H2NCH2CH2NH2)
- oxalate \[\ce{(C2O^{2-}_4)}\]
APPEARS IN
संबंधित प्रश्न
What is meant by unidentate ligand?
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
cis-[CrCl2(en)2]Cl
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Write the structures of compounds A, B and C in the following reactions
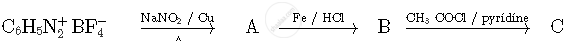
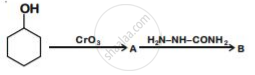
Write structures of compounds A and B of the following reaction :
Which of the following represents a chelate ligand?
Which of the following is non-ionizable?
In which of the following compounds, the Central metal atom/ion is in the lowest oxidation state?
When 0.1 mol \[\ce{CoCl3 (NH3)5}\] is treated with excess of \[\ce{AgNO3}\], 0.2 mol of \[\ce{AgCl}\] are obtained. The conductivity of solution will correspond to ______.
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Which one of the following does not achieve an octet of electrons in the central atom?
The oxidation state of Fe in the brown ring complex [F3(H2O)5NO]SO4 is
Ethylene diamine tetraacetate (EDTA) ion is ______.
Ethylene diaminetetraacetate (EDTA) ion is ______
How many ions are produced from the complex Co(NH3)6]Cl2 in solution?
Does ionization isomer for the following compound exist? Justify your answer.
\[\ce{Hg[Co(SCN)4]}\]
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Homoleptic
What is meant by didentate ligand?
