Advertisements
Advertisements
प्रश्न
What is meant by ambidentate ligand?
उत्तर
Ligand which has two different donor atoms and either of the two ligetes in the complex is called ambidentate ligand.
APPEARS IN
संबंधित प्रश्न
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
cis-[CrCl2(en)2]Cl
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
What happens when PCl5 is heated? Write the equations involved.
Write structures of compounds A and B of the following reaction :
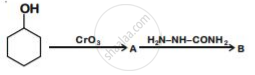
A group of atoms can function as a ligand only when:
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
Which of the following is non-ionizable?
When 0.1 mol \[\ce{CoCl3 (NH3)5}\] is treated with excess of \[\ce{AgNO3}\], 0.2 mol of \[\ce{AgCl}\] are obtained. The conductivity of solution will correspond to ______.
Which of the following complexes are homoleptic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Co(NH3)4 Cl2]^{+}}\]
(iii) \[\ce{[Ni(CN)4]^{2-}}\]
(iv) \[\ce{[Ni(NH3)4Cl2]}\]
A complex of the type \[\ce{[M(AA)2X2]^{n+}}\] is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
Assertion: \[\ce{Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2}\] are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
Oxidation number of cobalt in K[Co(CO)4] is
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
What are Homoleptic complexes?
Total sodium ions which are present in one formula unit of sodium ethane-1, 2-diaminetetraacetatochromate (II) and sodium hexanitrito cobaltate (III) are ______.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
In the complex ion \[\ce{[CoCl(en)2ONO]+}\], the coordination number and the oxidation number of the central metal ion are ______ and ______.
Give two examples of ambidentate ligand.
