Advertisements
Advertisements
Question
What is meant by ambidentate ligand?
Solution
Ligand which has two different donor atoms and either of the two ligetes in the complex is called ambidentate ligand.
APPEARS IN
RELATED QUESTIONS
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
[Mn(H2O)6]SO4
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
Write the structures of compounds A, B and C in the following reactions
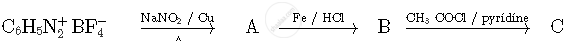
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
What is the coordination number of chromium in \[\ce{[Cr(NH3)2(H2O2)2]Cl3}\]?
The correct \[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2Cl2]}\] is ______.
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
Assertion: \[\ce{Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2}\] are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
The most stable ion is:-
Given below are two statements.
- Statement I: In CuSO4 · 5H2O, Cu-O bonds are present.
- Statement II: In CuSO4 · 5H2O, ligands coordinating with Cu(II) ion are O- and S based ligands.
In light of the above statement, choose the correct answer from the options given below.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
Does ionization isomer for the following compound exist? Justify your answer.
\[\ce{Hg[Co(SCN)4]}\]
Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Ligand
