Advertisements
Advertisements
प्रश्न
Give two examples of didentate ligand.
उत्तर
- Ethane-1, 2-diamine (H2NCH2CH2NH2)
- oxalate \[\ce{(C2O^{2-}_4)}\]
APPEARS IN
संबंधित प्रश्न
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
K3[Co(C2O4)3]
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
How is Benzonitrile converted to Benzophenone?
What happens when PCl5 is heated? Write the equations involved.
Write structures of compounds A, B and C in of the following reactions
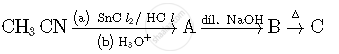
Write the IUPAC name of the following complex : [Co(NH3)5(CO3)]Cl.
Write the structures of compounds A, B and C in the following reactions:
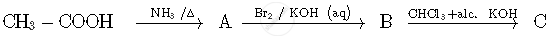
The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Which of the following is an ionic ligand?
Which one of the following ligands forms a chelate?
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
The oxidation number d-arbitral occupation and co-ordination number of Cr in the complex cis [Cr(en)2Cl2]Cl are respectively.
The most stable ion is:-
The correct order of the spin-only magnetic moment of metal ions in the following low spin complexes,
\[\ce{[V(CN)6]^{4-}, [Fe(CN)6]^{4-}, [Ru(NH3)6]^{3+} and [Cr(NH3)6]^2}\] is:
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Homoleptic
What is meant by didentate ligand?
