Advertisements
Advertisements
प्रश्न
What is meant by didentate ligand?
उत्तर
A didentate ligand, also known as a bidentate ligand, is a ligand that can form coordination bonds to a metal ion using two donor atoms. These donor atoms are typically separated by a specific number of atoms in the ligand's structure, forming a chelate complex.
संबंधित प्रश्न
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Write applications of co-ordination compounds in medicine and electroplating.
Write structures of compounds A, B and C in of the following reactions
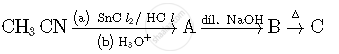
Write the IUPAC name of the following complex : [Co(NH3)5(CO3)]Cl.
Complete the following reactions
NH3+3Cl2(excess) ---->
Write the structures of compounds A, B and C in the following reactions:
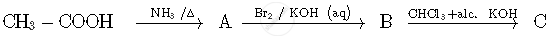
Write the structures of compounds A, B and C in the following reactions
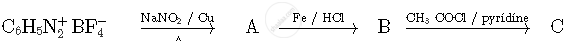
The oxidation number of Fe in K4[Fe(CN)6] is ____________.
In which of the following compounds the oxidation state of the nickel atom is 0?
What is the coordination number of chromium in \[\ce{[Cr(NH3)2(H2O2)2]Cl3}\]?
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
In which of the following compounds, the oxidation number of iodine is fractional?
Which one of the following ligands forms a chelate?
The oxidation number d-arbitral occupation and co-ordination number of Cr in the complex cis [Cr(en)2Cl2]Cl are respectively.
The complex which has no d electrons in the central atom is:-
In the complex ion \[\ce{[CoCl(en)2ONO]+}\], the coordination number and the oxidation number of the central metal ion are ______ and ______.
Explain the following, giving two examples:
Coordination polyhedron
Explain the following, giving two examples:
Homoleptic
