Advertisements
Advertisements
प्रश्न
Write applications of co-ordination compounds in medicine and electroplating.
उत्तर
Applications of coordination compounds:
1) In medicine:
- Cisplatin [PtCl2(NH3)2] is useful in treatment of cancer.
- EDTA is useful in treatment of poisoning by lead.
2) In electroplating
Stable complexes with very small dissociation in solution are used for electroplating with silver and gold.
- The complex K[Ag(CN)2] is used for electroplating with silver
- The complex K[Au(CN)2] is used for electroplating with gold
APPEARS IN
संबंधित प्रश्न
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Write the structure and IUPAC names of all the metamers represented by formula C4H10
What is meant by unidentate ligand?
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
K3[Co(C2O4)3]
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Complete the following reactions
NH3+3Cl2(excess) ---->
Write the IUPAC names of the following coordination compounds:
[Cr(NH3)4(H2O)2]Cl3
Write the IUPAC names of the following coordination compounds:
`[PtCl_2(NH_3)_4][PtCl_4]`
The ligand triethylenetetramine is _______.
Ligand (en) is an example of ___________.
What is the coordination number of chromium in \[\ce{[Cr(NH3)2(H2O2)2]Cl3}\]?
Which of the following is non-ionizable?
Which of the following complexes formed by \[\ce{Cu^2+}\] ions is most stable?
The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
Which of the following complexes are homoleptic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Co(NH3)4 Cl2]^{+}}\]
(iii) \[\ce{[Ni(CN)4]^{2-}}\]
(iv) \[\ce{[Ni(NH3)4Cl2]}\]
A complex of the type \[\ce{[M(AA)2X2]^{n+}}\] is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Match the complex ions given in Column I with the colours given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Colour) |
| A. \[\ce{[Co(NH3)6]^{3+}}\] | 1. Violet |
| B. \[\ce{[Ti(H2O)6]^{3+}}\] | 2. Green |
| C. \[\ce{[Ni(H2O)6]^{2+}}\] | 3. Pale blue |
| D. \[\ce{(Ni(H2O)4 (en)]^{2+} (aq)}\] | 4. Yellowish orange |
| 5. Blue |
Which of the following is an ionic ligand?
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
Metal attached with EDTA in an octahedral complex, has coordination number ______.
Why chelate complexes are more stable than complexes with unidentate ligands?
What are Heteroleptic complexes?
The following ligand is:
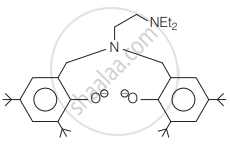
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Ligand
Give two examples of didentate ligand.
