Advertisements
Advertisements
Question
What is meant by didentate ligand?
Solution
A didentate ligand, also known as a bidentate ligand, is a ligand that can form coordination bonds to a metal ion using two donor atoms. These donor atoms are typically separated by a specific number of atoms in the ligand's structure, forming a chelate complex.
RELATED QUESTIONS
Explain the following, giving two examples:
Coordination entity
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
cis-[CrCl2(en)2]Cl
How is Benzonitrile converted to Benzophenone?
What happens when PCl5 is heated? Write the equations involved.
Write structures of compounds A, B and C in of the following reactions
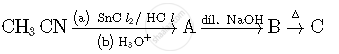
Write structures of compounds A and B of the following reaction :

Ligand (en) is an example of ___________.
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
The coordination number of the central ion may be obtained from:
Which of the following complexes are homoleptic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Co(NH3)4 Cl2]^{+}}\]
(iii) \[\ce{[Ni(CN)4]^{2-}}\]
(iv) \[\ce{[Ni(NH3)4Cl2]}\]
Oxidation number of cobalt in K[Co(CO)4] is
According to IUPAC nomenclatures, sodium nitroprusside is named as
What are Homoleptic complexes?
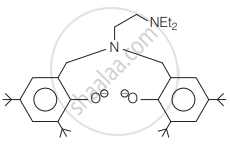
The following ligand is:
The correct order of the spin-only magnetic moment of metal ions in the following low spin complexes,
\[\ce{[V(CN)6]^{4-}, [Fe(CN)6]^{4-}, [Ru(NH3)6]^{3+} and [Cr(NH3)6]^2}\] is:
Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
Explain the following, giving two examples:
Ligand
Give two examples of unidentate ligand.
What is meant by ambidentate ligand?
