Advertisements
Advertisements
Question
What happens when PCl5 is heated? Write the equations involved.
Solution
When heated, PCl5 sublimes but decomposes on stronger heating.
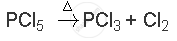
APPEARS IN
RELATED QUESTIONS
Write IUPAC names of the following compounds:
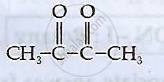
Which of the following represents a chelate ligand?
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Which one of the following does not achieve an octet of electrons in the central atom?
The oxidation state of Fe in the brown ring complex [F3(H2O)5NO]SO4 is
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
The complex which has no d electrons in the central atom is:-
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
