Advertisements
Advertisements
Question
Give two examples of unidentate ligand.
Solution
- Cl−
- H2O
- NH3
APPEARS IN
RELATED QUESTIONS
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
cis-[CrCl2(en)2]Cl
What happens when PCl5 is heated? Write the equations involved.
Following compounds are given to you :
2-Bromopentane, 2-Bromo-2-methylbutane, 1-Bromopentane
1) Write the compound which is most reactive towards SN2 reaction.
2) Write the compound which is optically active.
3) Write the compound which is most reactive towards β-elimination reaction.
Write the structures of compounds A, B and C in the following reactions:
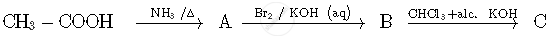
Write the IUPAC names of the following coordination compounds:
[Cr(NH3)4(H2O)2]Cl3
Write structures of compounds A and B of the following reaction :

Which of the following represents a chelate ligand?
A group of atoms can function as a ligand only when:
In which of the following compounds the oxidation state of the nickel atom is 0?
When 0.1 mol \[\ce{CoCl3 (NH3)5}\] is treated with excess of \[\ce{AgNO3}\], 0.2 mol of \[\ce{AgCl}\] are obtained. The conductivity of solution will correspond to ______.
Arrange the following complexes in the increasing order of conductivity of their solution:
[Co(NH3)3Cl3], [Co(NH3)4Cl2]Cl, [Co(NH3)6]Cl3, [Cr(NH3)5Cl]Cl2
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
In which of the following compounds, the oxidation number of iodine is fractional?
The most stable ion is:-
According to IUPAC nomenclatures, sodium nitroprusside is named as
The one that will show optical activity is: (en = ethane 1, 2-diamine)
In the complex ion \[\ce{[CoCl(en)2ONO]+}\], the coordination number and the oxidation number of the central metal ion are ______ and ______.
What is meant by didentate ligand?
