Advertisements
Advertisements
प्रश्न
How is Benzonitrile converted to Benzophenone?
How is benzophenone prepared from benzonitrile?
उत्तर
Benzonitrile reacts with phenyl magnesium bromide in equimolecular proportion in the presence of dry ether to give an adduct, which on acid hydrolysis gives benzophenone

APPEARS IN
संबंधित प्रश्न
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
[Mn(H2O)6]SO4
Amongst the following, the most stable complex is:
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
IUPAC name of the following compound is

(a) 3 - Bromo- 3, 4- dimethylheptane
(b) 3, 4- dimethyl - 3- bromoheptane
(c) 5- Bromo- 4, 5- dimethylheptane
(d) 4, 5- dimethyl- 5- bromoheptane
Write structures of compounds A, B and C in of the following reactions
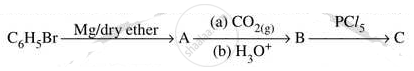
Write the IUPAC name of the following complex : [Co(NH3)5(CO3)]Cl.
The ligand triethylenetetramine is _______.
Write structures of compounds A and B of the following reaction :
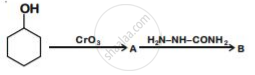
The coordination number of Cr in [Cr(NH3)3(H2O)3]Cl3 is ___________.
Which of the following is non-ionizable?
In which of the following compounds, the Central metal atom/ion is in the lowest oxidation state?
Which of the following complexes are homoleptic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Co(NH3)4 Cl2]^{+}}\]
(iii) \[\ce{[Ni(CN)4]^{2-}}\]
(iv) \[\ce{[Ni(NH3)4Cl2]}\]
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Which one of the following does not achieve an octet of electrons in the central atom?
Oxidation number of cobalt in K[Co(CO)4] is
The nature of hybridisation in the ammonia molecule is
The oxidation number d-arbitral occupation and co-ordination number of Cr in the complex cis [Cr(en)2Cl2]Cl are respectively.
Which of the following ligands can exhibit linkage isomerism?
Metal attached with EDTA in an octahedral complex, has coordination number ______.
What is a chelate complex? Give one example.
Which of the following species cannot act as a ligand? Give reason.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Coordination polyhedron
Give two examples of ambidentate ligand.
