Advertisements
Advertisements
Question
Amongst the following, the most stable complex is:
Options
[Fe(H2O)6]3+
[Fe(NH3)6]3+
[Fe(C2O4)3]3−
[FeCl6]3−
Solution
[Fe(C2O4)3]3−
Explanation:
\[\ce{C2O^{2-}_4}\] is a bidentate ligand, it forms the most stable complex.
APPEARS IN
RELATED QUESTIONS
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Write the structure and IUPAC names of all the metamers represented by formula C4H10
What is meant by unidentate ligand?
Predict the co-ordination No. of cs+ ion if `r_(Cs)^+` = 1.69Å and `r_(Cl)^-` = 1.81Å.
Write IUPAC names of the following compounds
Write applications of co-ordination compounds in medicine and electroplating.
Write the IUPAC names of the following coordination compounds:
[Cr(NH3)4(H2O)2]Cl3
The oxidation number of Fe in K4[Fe(CN)6] is ____________.
What is the coordination number of chromium in \[\ce{[Cr(NH3)2(H2O2)2]Cl3}\]?
Which of the following is non-ionizable?
The correct \[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2Cl2]}\] is ______.
Arrange the following complexes in the increasing order of conductivity of their solution:
[Co(NH3)3Cl3], [Co(NH3)4Cl2]Cl, [Co(NH3)6]Cl3, [Cr(NH3)5Cl]Cl2
Match the complex ions given in Column I with the colours given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Colour) |
| A. \[\ce{[Co(NH3)6]^{3+}}\] | 1. Violet |
| B. \[\ce{[Ti(H2O)6]^{3+}}\] | 2. Green |
| C. \[\ce{[Ni(H2O)6]^{2+}}\] | 3. Pale blue |
| D. \[\ce{(Ni(H2O)4 (en)]^{2+} (aq)}\] | 4. Yellowish orange |
| 5. Blue |
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
Which one of the following does not achieve an octet of electrons in the central atom?
What are Homoleptic complexes?
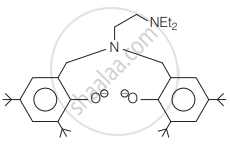
The following ligand is:
The correct order of the spin-only magnetic moment of metal ions in the following low spin complexes,
\[\ce{[V(CN)6]^{4-}, [Fe(CN)6]^{4-}, [Ru(NH3)6]^{3+} and [Cr(NH3)6]^2}\] is:
Explain the following, giving two examples:
Homoleptic
