Advertisements
Advertisements
Question
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Solution
- Ammonia - monodentate
- Carbon monoxide - monodentate
- Ethylene diamine - polydentate
- Ethylene diamine tetra acetate ion - polydentate
APPEARS IN
RELATED QUESTIONS
Write IUPAC names of the following compounds:
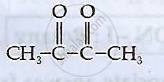
Write structures of compounds A, B and C in of the following reactions
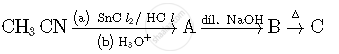
Write IUPAC name of the following Complex [Cr(NH3)3Cl3]
Write the structures of compounds A, B and C in the following reactions:
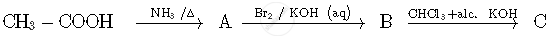
Write the IUPAC names of the following coordination compounds:
`[PtCl_2(NH_3)_4][PtCl_4]`
The ligand triethylenetetramine is _______.
Which of the following represents a chelate ligand?
A group of atoms can function as a ligand only when:
In which of the following compounds the oxidation state of the nickel atom is 0?
Which of the following is non-ionizable?
Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
A complex of the type \[\ce{[M(AA)2X2]^{n+}}\] is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Match the complex ions given in Column I with the colours given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Colour) |
| A. \[\ce{[Co(NH3)6]^{3+}}\] | 1. Violet |
| B. \[\ce{[Ti(H2O)6]^{3+}}\] | 2. Green |
| C. \[\ce{[Ni(H2O)6]^{2+}}\] | 3. Pale blue |
| D. \[\ce{(Ni(H2O)4 (en)]^{2+} (aq)}\] | 4. Yellowish orange |
| 5. Blue |
Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code:
| Column I (Compound) | Column II (Oxidation state of Co) |
| A. \[\ce{[Co(NCS)(NH3)5](SO3)}\] | 1. + 4 |
| B. \[\ce{[Co(NH3)4 CL2]SO4}\] | 2. 0 |
| C. \[\ce{Na4[Co(S2O3)3]}\] | 3. + 1 |
| D. \[\ce{[Co2(CO)8]}\] | 4. + 2 |
| 5. + 3 |
Which one of the following does not achieve an octet of electrons in the central atom?
Which of the following is an ionic ligand?
Metal attached with EDTA in an octahedral complex, has coordination number ______.
Ethylene diaminetetraacetate (EDTA) ion is ______
What are Heteroleptic complexes?
The following ligand is:
The correct order of the spin-only magnetic moment of metal ions in the following low spin complexes,
\[\ce{[V(CN)6]^{4-}, [Fe(CN)6]^{4-}, [Ru(NH3)6]^{3+} and [Cr(NH3)6]^2}\] is:
What is a chelate complex? Give one example.
Which of the following species cannot act as a ligand? Give reason.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
Is the central metal atom in coordination complexes a Lewis acid or a Lewis base? Explain.
Explain the following, giving two examples:
Ligand
Explain the following, giving two examples:
Coordination number
Explain the following, giving two examples:
Heteroleptic
Give two examples of didentate ligand.
