HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2013-2014
Date: October 2013
Advertisements
The Arrhenius equation is_________________ .
(a)`k=Ae^(RT/E_a)`
(b)`A=ke^(-E_a/(RT))`
(c)`k=Ae^(-(RT)/E_a)`
(d)`k=Ae^(-E_a/(RT))`
Chapter: [0.05] Chemical Kinetics
If the enthalpy of vaporisation of water at 100oC is 186.5 J.mol-1, the entropy of vaporization will be_____________ .
(a) 4.0 J . K-1. mol-1
(b) 3.0 J . K-l. mol-1
(c) 1.5 J - K-1. mol-1
(d) 0.5 J . K-l. mol-1
Chapter: [0.03] Chemical Thermodynamics and Energetic
The atomicity of sulphur in orthorhombic sulphur is______________ .
(a) 8
(b) 6
(c) 4
(d) 2
Chapter: [7.02] Group 16 Elements
The major binding force in diamond is_______________ .
(a) Covalent bond
(b) Ionic bond
(c) Metallic bond
(d) Co-ordinate covalent bond
Chapter: [0.01] Solid State [0.01] Solid State
The boiling point of water at high altitude is low. because________________ .
(a) the temperature is low.
(b) the atmospheric pressure is low.
(c) the temperature is high.
(d) the atmospheric pressure is high
Chapter: [0.02] Solutions and Colligative Properties
The molar conductivity of cation and anion of salt BA are 180 and 220 mhos respectively. The molar conductivity of salt BA at infinite dilution is_____________ .
(a) 90 mhos.cm2
(b) 110 mhos.cm2.mol-1
(c) 200 mhos.cm2.mol-1
(d) 400 mhos.cm2.mol-1
Chapter: [0.04] Electrochemistry
What is the process in which concentrated ore is reduced to the corresponding metal by heating at high temperature with a reducing agent?
(a) Polling
(b) Pyrometallurgy
(c) Hydrometallurgy
(d) Calcination
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Describe anomalous behaviour of oxygen as compared with other elements of group 16 with reference to -
(a) Magnetic property
(b) Oxidation state
(c) Hydrides
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.02] Group 16 Elements
What is the value of for the following reaction at 298 K -
6CO2+ 6H20(l) → C6H1206(s) + 602(g),
Given that: ΔG° = 2879 kj mol-1, AS = -210 JK-1 mol-1.
Chapter: [0.04] Electrochemistry
Sucrose decomposes in acid solution to give glucose and fructose according to the first order rate law. The half life of the reaction is 3 hours. Calculate fraction of sucrose which will remain after 8 hours.
Chapter: [0.05] Chemical Kinetics [0.06] Chemical Kinetics
A solution containing 0.73 g of camphor (molar mass 152 g . mol-1) in 36.8 g of acetone (boiling point 56.3°C) boils at 56.55°C. A solution of 0.564 g of unknown compound in the same weight of acetone boils at 56.46oC. Calculate the molar mass of the unknown compound.
Chapter: [0.02] Solutions and Colligative Properties
Describe triclinic crystal lattice with the help of a diagram.
Chapter: [0.01] Solid State
Write any four applications of electrochemical series
Chapter: [0.04] Electrochemistry
Advertisements
State and explain Hess’s law of constant heat summation.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Distinguish between Order and Molecularity of reaction.
Chapter: [0.06] Chemical Kinetics
With the help of the equation ΔG° = - nFEocell. Explain that cell potential is an intensive property.
Chapter: [0.04] Electrochemistry
Describe the laboratory method of preparation of ammonia
Chapter: [7.01] Group 15 Elements
Define van’t Hoff factor.
Chapter: [0.02] Solutions and Colligative Properties
How van’t Hoff factor is related to the degree of dissociation?
Chapter: [0.02] Solutions and Colligative Properties
Write chemical formulae of the following ores :
(a) Calamine
(b) Haematite
(c) Magnetite
(d) Corundum
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Write the reactions involved in extraction of silver from its ore by leaching process.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Derive the equation : W = - PextAV
Chapter: [0.03] Chemical Thermodynamics and Energetic
A unit cell of iron crystal has edge length 288 pm and density 7.86 g.cm-3. Find the number of atoms per unit cell and type of the crystal lattice.
Given : Molar mass of iron = 56 g.mol-1; Avogadro's number NA = 6.022 x 1023.
Chapter: [0.01] Solid State
Define Cryoscopic constant.
Chapter: [0.02] Solutions and Colligative Properties
What is the action of hot/concentrated nitric acid on - Arsenic
Chapter: [7.01] Group 15 Elements
What is the action of hot/concentrated nitric acid on - Antimony.
Chapter: [7.01] Group 15 Elements
Draw the structure of: Orthophosphoric acid
Chapter: [7.01] Group 15 Elements
Draw the structure of: Pyrophosphoric acid
Chapter: [7.01] Group 15 Elements
How much electricity in terms of Faraday is required to produce 20 g of \[\ce{Ca}\] from molten \[\ce{CaCl2}\]?
(Given: Molar mass of Calcium is 40 g mol−1.)
Chapter: [0.04] Electrochemistry
How much electricity in terms of Faraday is required to produce 40.0 g of \[\ce{Al}\] from molten \[\ce{Al2O3}\]?
(Given: Molar mass of Aluminium is 27 g mol−1.)
Chapter: [0.04] Electrochemistry
Which of the followings is a trihydric alcohol ?
(a) n-propyl alcohol
(b) Glycerol
(c) Glycol
(d) Glycine
Chapter: [11.01] Alcohols
Alkyl halides are -
(a) Mono halogen derivatives of alkanes
(b) Di halogen derivatives of alkanes
(c) Tri halogen derivatives of alkanes
(d) Tetra halogen derivatives of alkanes
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
Mohr’s Salt is -
(a) Ferrous ammonium sulphate
(b) Ferrous sulphate
(c) Ammonium sulphate
(d) Ferric sulphate
Chapter: [0.09] Coordination Compounds
Which of the following is polyamide ?
(a) Teflon
(b) Nylon 6,6
(c) Terylene
(d) Bakelite
Chapter: [0.15] Polymers
Vitamin ‘C’ belongs to the class of—
(a) Vitamins of aliphatic series
(b) Vitamins of aromatic series
(c) Vitamins of alicyclic series
(d) Vitamins of heterocyclic series
Chapter: [14.03] Vitamins
What is the IUPAC name of 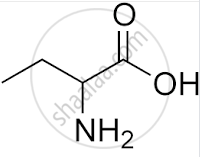
α - Amino butanoic acid
2 - Amino butyric acid
α - Aminobutyric acid
2 - Amino butanoic acid
Chapter: [0.09] Coordination Compounds
Advertisements
Which among the following molecular formulae represents urotropine?
(a) C6H12N4
(b) C6H24H4
(c) C6H12N4O2
(d) C6H24N4O2
Chapter: [13.01] Amines
Write the structures of - 3-chloro-3-ethylhex-1-ene
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
Write the structures of -1-Iodo-2, 3-dimethylbutane
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
Write the structures of - 1, 3, 5 - tribromobenzene
Chapter: [10.01] Haloalkanes [10.02] Haloarenes
What is the action of acidified potassium dichromate on - SO2
Chapter: [8.01] D-block Elements
What is the action of acidified potassium dichromate on - KI
Chapter: [8.01] D-block Elements
Draw structure of dichromate ion
Chapter: [8.01] D-block Elements
Describe laboratory method for preparation of glucose.
Chapter: [14.01] Carbohydrates
Write the reaction that indicates the presence of -CHO group in glucose
Chapter: [14.01] Carbohydrates
What will be the action of the mixture of sodium nitrite and dilute hydrochloric acid on ethyl amine
Chapter: [13.01] Amines
What will be the action of the mixture of sodium nitrite and dilute hydrochloric acid on aniline
Chapter: [13.01] Amines
What will be the action of the mixture of sodium nitrite and dilute hydrochloric acid on triethyl amine
Chapter: [13.01] Amines
What are chemical twins? Write ‘two’ examples.
Chapter: [8.02] F-block Elements
Explain the term Antiseptics
Chapter: [16.01] Chemicals in Medicines
Explain trem Analgesics.
Chapter: [16.01] Chemicals in Medicines
Draw the simple Fisher projection formulae of D - (+) - glucose and D - (-) - fructose
Chapter: [14.01] Carbohydrates
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Chapter: [0.09] Coordination Compounds
State and explain Markownikoff's rule with suitable example
Chapter: [0.1] Halogen Derivatives [10.01] Haloalkanes
How are propan-1-amine and propan-2-amine prepared from oxime?
Chapter: [0.13] Amines [13.01] Amines
Identify ‘A' and ‘B’ in the following reaction :
C6H5MgBr + C02 `(`> ‘A’ `(PCl_5)/()`> ‘B’
Chapter: [12.02] Carboxylic Acids
What is the action of the following reagents on phenol - Bromine in CS2 at low temperature.
Chapter: [11.02] Phenols
What is the action of the following reagents on phenol - H2SO4 at room temperature.
Chapter: [11.02] Phenols
Write the structure and IUPAC names of all the metamers represented by formula C4H10
Chapter: [0.09] Coordination Compounds
Write balanced chemical equations for action of ammonia on - formaldehyde
Chapter: [12.01] Aldehydes and Ketones
Write balanced chemical equations for action of ammonia on - acetaldehyde
Chapter: [12.01] Aldehydes and Ketones
Write balanced chemical equations for action of ammonia on - acetone
Chapter: [12.01] Aldehydes and Ketones
Write ‘four’ characteristics of co-ordinate complex ion.
Chapter: [0.09] Coordination Compounds
Write any ‘two' uses of terylene.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Write ‘four’ physical methods of preserving food materials
Chapter: [16.02] Chemicals in Food
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2013 - 2014
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2014 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
