Advertisements
Advertisements
Question
Draw the structure of: Orthophosphoric acid
Solution
Molecular Formula: H3O4P or H3PO4
Structure:
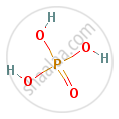
APPEARS IN
RELATED QUESTIONS
The hybridisation of phosphorus in phosphorus pentachloride is _______.
(A) dsp3
(B) sp3 d
(C) d2 sp3
(D) sp3 d2
Draw structure and write geometry of PCl3 and PCl5.
Draw the structures of the following molecules: (HPO3)3
Draw the structure of: Pyrophosphoric acid
Draw the structure of H4P2O6 hypophosphoric acid
Write balanced chemical equations for Phosphorus reacts with magnesium
Write balanced chemical equations for Flowers of sulphur boiled with calcium hydroxide
Write balanced chemical equations for Action of ozone on hydrogen peroxide.
\[\begin{array}{cc}
\phantom{.}\ce{OH}\\
|\phantom{..}\\
\ce{O=P-OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
is the structure of:-
Pentavalence in phosphorus is more stable when compared to that of nitrogen even though they belong to the same group. This is due to ____________.
Which of the following elements does not form stable diatomic molecules?
Which gas cannot be collected over water?
On heating with concentrated \[\ce{NaOH}\] solution in an inert atmosphere of \[\ce{CO2}\], white phosphorus gives a gas. Which of the following statement is incorrect about the gas?
In the preparation of compounds of Xe, Bartlett had taken \[\ce{O^{+}_2 Pt F^{-}_6}\] as a base compound. This is because ______.
One mole of calcium phosphide on reaction with excess water gives ______
What is the basicity of H3PO4?
