Advertisements
Advertisements
Question
How are propan-1-amine and propan-2-amine prepared from oxime?
Solution
The basic reaction to prepare amine from oxime is as follows:

If R1 = H and R2 = CH3CH2 then the product will be propane-1-amine.
If R1 and R2 both are CH3 then the product will be propane-2-amine.
APPEARS IN
RELATED QUESTIONS
Give the structures of A, B and C in the following reactions :
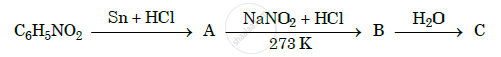
Give the structures of A, B and C in the following reactions :
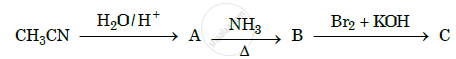
Accomplish the following conversions Nitrobenzene to benzoic acid
Accomplish the following conversions: Benzyl chloride to 2-phenylethanamine
Accomplish the following conversions: Benzamide to toluene
Give the structures of A, B and C in the following reaction:

Give the structures of A, B and C in the following reaction:
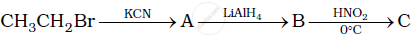
Give the structures of A, B and C in the following reaction.

Write the reactions of aromatic with nitrous acid.
Write the reactions of aliphatic primary amines with nitrous acid.
Explain the mechanism of action of hydroiodic acid on 3-methylbutan-2-ol.
Give the structures of A, B and C in the following reactions :
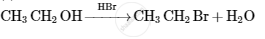
Give the structures of A, B and C in the following reactions :

Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Choose the most correct option.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
Name the process of breaking C-X bond by ammonia in preparation of amines.
Explain Hoffmann’s exhaustive alkylation with suitable reactions.
Acetamide on reduction using Na/C2H5OH gives ____________.
Identify the product 'A' in the following reaction.
\[\ce{Aniline ->[(CH3CO)2O][Pyridine] A}\]
The end product C of the following reaction is
\[\ce{C2H5NH2 ->[HNO2] A ->[PCl5] B ->[NH3][Alcohol] C}\]
Which of the following amines exhibits maximum degree of intermolecular hydrogen bonding?
____________ can be prepared exclusively by Gabriel phthalimide synthesis.
What product is formed when \[\ce{R - C ≡ N}\] is hydrolysed?
\[\ce{CH3-CN ->[Na/C2H5OH]}\]
The product formed is ____________.
Which of the following reactions is appropriate for converting benzamide to aniline?
Identify 'A' and 'B' in the following conversions.
\[\ce{CH3 - I ->[Alc. KCN][\Delta] A ->[Na/C2H5OH] B}\]
Which of the following reactions does NOT yield an amine?
Benzylamine may be alkylated as shown in the following equation:
\[\ce{C6H5CH2NH2 + R - X -> C6H5CH2NHR}\]
Which of the following alkylhalides is best suited for this reaction through SN1 mechanism?
Amongst the given set of reactants, the most appropriate for preparing 2° amine is ______.
Hoffmann Bromamide Degradation reaction is shown by ______.
Which of the following compounds is the weakest Brönsted base?
Which of the following methods of preparation of amines will give same number of carbon atoms in the chain of amines as in the reactant?
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) \[\ce{Sn/HCl}\]
(ii) \[\ce{Fe/HCl}\]
(iii) \[\ce{H2 - Pd}\]
(iv) \[\ce{Sn/NH4OH}\]
The reagents that can be used to convert benzenediazonium chloride to benzene are:
(i) \[\ce{SnCl2/HCl}\]
(ii) \[\ce{CH3CH2OH}\]
(iii) \[\ce{H3PO2}\]
(iv) \[\ce{LiAlH4}\]
How will you carry out the following conversion?
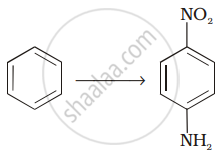
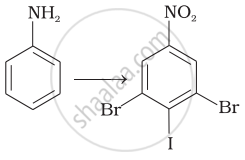
How will you carry out the following conversions?
Assertion: Hoffmann’s bromamide reaction is given by primary amines.
Reason: Primary amines are more basic than secondary amines.
Describe Gabriel's phthalimide synthesis. (Give reaction)
The compound X is which of the following?
\[\ce{CH3CN ->[Na + C2H5OH] x}\]
Acetamide and ethyl amide can be distinguished by reacting with.
Which of the following compound is expected to be most basic?
C6H5CONHCH3 can be converted into C6H5CH2NHCH3 by:-
A compound 'A' on reduction with iron scrap and hydrochloric acid gives compound 'B' with molecular formula C6H7N. Compound 'B' on reaction with CHCl3 and alcoholic KOH produces an obnoxious smell of carbylamine due to the formation of 'C'. Identify 'A', 'B' and 'C' and write the chemical reactions involved.
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
Which of the following reaction DOES NOT involve Hoffmann bromamide degradation?
Methyl amine on reaction with chloroform in the presence of NaOH gives ______.
What is the IUPAC name of \[\ce{(CH3)2 - N - CH3}\]?
Amides can be converted into amines by the reaction named ______.
Write short note on the following:
Ammonolysis
Identify the compo ds A and B in the following reactions:
\[\ce{A ->[Nitrating mixture] B ->[(i) Sn/cone. HCI][(ii) NaOH] Aniline}\]
Write a short note on the following:
Ammonolysis
Write the name of reduction product formed when ethyl cyanide is treated with sodium and alcohol.
Write short note on the following:
Ammonolysis
Assertion: Amimonolysis of alkyl halides involves the reaction between alkyl halides and alcoholic ammonia.
Reason: Ammonolysis of alkyl halides produces secondary amines only.
