Advertisements
Advertisements
Question
Benzylamine may be alkylated as shown in the following equation:
\[\ce{C6H5CH2NH2 + R - X -> C6H5CH2NHR}\]
Which of the following alkylhalides is best suited for this reaction through SN1 mechanism?
Options
\[\ce{CH3Br}\]
\[\ce{C6H5Br}\]
\[\ce{C6H5CH2Br}\]
\[\ce{C2H5Br}\]
Solution
\[\ce{C6H5CH2Br}\]
Explanation:
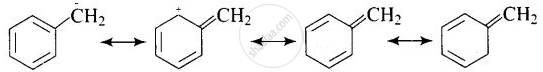
SN1 reaction occurs in two steps. In first step R – X bond is broken to produce a carbocation which is attacked by nucleophile. The greater the stability of carbocation, the greater will be the rate of reaction. Benzylic halides show high reactivity towards SN1 reaction.
APPEARS IN
RELATED QUESTIONS
Give the structures of A, B and C in the following reaction:
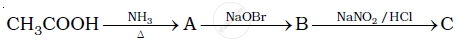
Answer in one sentence.
Predict the product of the following reaction.
\[\ce{Nitrobenzene ->[Sn/conc.HCl]?}\]
\[\ce{CH3-CN ->[Na/C2H5OH]}\]
The product formed is ____________.
Quaternary ammonium salt is formed:
Amongst the following, the strongest base in aqueous medium is ______.
Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
The best reagent for converting 2–phenylpropanamide into 2-phenylpropanamine is ______.
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
The compound X is which of the following?
\[\ce{CH3CN ->[Na + C2H5OH] x}\]
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
