Advertisements
Advertisements
Question
Which of the following is the weakest Brönsted base?
Options

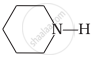
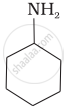
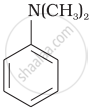
\[\ce{CH3NH2}\]
Solution

Explanation:
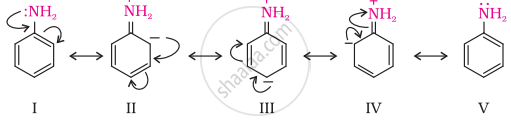
Due to delocalization of lone pair of electrons on the N-atom into the benzene ring, \[\ce{C6H5NH2}\] is the weakest base.
Resonating Structure of Aniline
APPEARS IN
RELATED QUESTIONS
Explain basic nature of amines.
Give the structures of A, B and C in the following reactions :

Give the structures of A, B and C in the following reactions :

Write structures of compounds A and B in each of the following reactions: 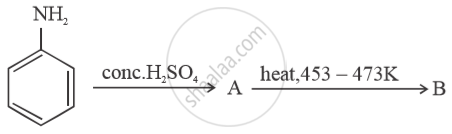
The following compound is called:
\[\begin{array}{cc}
\ce{CH3}\\
/\phantom{....}\\
\ce{CH3-N}\phantom{.............}\\
\backslash\phantom{....}\\
\phantom{.}\ce{CH3}
\end{array}\]
Which of the following is a 3° amine?
The correct IUPAC name for \[\ce{CH2 = CHCH2 NHCH3}\] is ______.
Give the structure of ‘A’ in the following reaction.
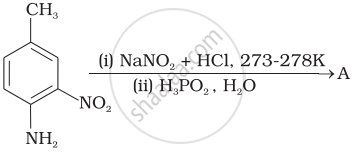
What is the structure and IUPAC name of the compound, allylamine?
Write down the IUPAC name of